BRAF V600E immunohistochemistry is reliable in primary and metastatic colorectal carcinoma regardless of treatment status and shows high intratumoral homogeneity
- PMID: 24921639
- PMCID: PMC4167743
- DOI: 10.1097/PAS.0000000000000263
BRAF V600E immunohistochemistry is reliable in primary and metastatic colorectal carcinoma regardless of treatment status and shows high intratumoral homogeneity
Abstract
In colorectal carcinoma the evaluation of BRAF mutation status is increasingly being performed given its utility as a prognostic and predictive biomarker. However, there are conflicting reports of the sensitivity and specificity of BRAF V600E immunohistochemistry (IHC), and little is known about its reliability in tissues collected from metastatic sites or after chemotherapy, radiation therapy and/or targeted therapy. The degree of intratumoral staining heterogeneity is also not well established. We performed IHC for BRAF V600E (VE1) on 204 cases of colorectal carcinoma including 59 with the BRAF V600E mutation. These included primary (n=147) and metastatic/recurrent (n=57) tumors, collected before (n=133) or after (n=71) chemotherapy, radiation therapy and/or targeted therapy. Evaluation of a test cohort (39 cases) with knowledge of mutation status established a specific staining pattern for the mutation: diffuse cytoplasmic staining of near-uniform intensity, regardless of strength of staining. Using this pattern, pathologists at 3 levels of training independently performed blinded evaluation of the remaining cases. BRAF V600E staining was 96.3% sensitive and 98.5% specific for the mutation, including both pretreatment and posttreatment specimens. Fleiss κ for interobserver agreement was 0.96. Staining of whole sections of the BRAF mutants showed diffuse staining in all cases and uniform or near-uniform intensity in 91%. In 20 cases with both pretreatment and posttreatment specimens, there was 100% accuracy and agreement in staining between samples. We conclude that BRAF V600E IHC is reliable for the evaluation of mutational status in colorectal carcinoma regardless of site or prior treatment history, and staining shows a high degree of intratumoral homogeneity.
Conflict of interest statement
Figures

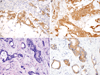
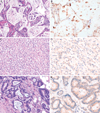

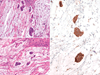
Similar articles
-
VE1 immunohistochemistry predicts BRAF V600E mutation status and clinical outcome in colorectal cancer.Oncotarget. 2015 Dec 8;6(39):41453-63. doi: 10.18632/oncotarget.6162. Oncotarget. 2015. PMID: 26496026 Free PMC article.
-
Detection of the BRAF V600E mutation in colon carcinoma: critical evaluation of the imunohistochemical approach.Am J Surg Pathol. 2014 Sep;38(9):1235-41. doi: 10.1097/PAS.0000000000000229. Am J Surg Pathol. 2014. PMID: 24832158 Free PMC article.
-
A mutant BRAF V600E-specific immunohistochemical assay: correlation with molecular mutation status and clinical outcome in colorectal cancer.Target Oncol. 2015 Mar;10(1):99-109. doi: 10.1007/s11523-014-0319-8. Epub 2014 May 27. Target Oncol. 2015. PMID: 24859797 Free PMC article.
-
Immunohistochemistry with Anti-BRAF V600E (VE1) Mouse Monoclonal Antibody is a Sensitive Method for Detection of the BRAF V600E Mutation in Colon Cancer: Evaluation of 120 Cases with and without KRAS Mutation and Literature Review.Pathol Oncol Res. 2019 Jan;25(1):349-359. doi: 10.1007/s12253-017-0344-x. Epub 2017 Nov 10. Pathol Oncol Res. 2019. PMID: 29127628 Free PMC article. Review.
-
BRAF V600E mutation-specific antibody: A review.Semin Diagn Pathol. 2015 Sep;32(5):400-8. doi: 10.1053/j.semdp.2015.02.010. Epub 2015 Feb 7. Semin Diagn Pathol. 2015. PMID: 25744437 Review.
Cited by
-
Deciphering the Role of BRAFV600E Immunohistochemistry in Breast Lesions: A Comprehensive Review.Cureus. 2024 Jul 18;16(7):e64872. doi: 10.7759/cureus.64872. eCollection 2024 Jul. Cureus. 2024. PMID: 39156294 Free PMC article. Review.
-
BRAF V600E Expression by Immunohistochemistry in Colon Cancer and Clinico-pathologic Features Associated with BRAF-Mutated Colonic Cancers in Mexican Patients.J Gastrointest Cancer. 2020 Mar;51(1):35-40. doi: 10.1007/s12029-018-00191-9. J Gastrointest Cancer. 2020. PMID: 30618001
-
Immunohistochemistry for predictive biomarkers in non-small cell lung cancer.Transl Lung Cancer Res. 2017 Oct;6(5):570-587. doi: 10.21037/tlcr.2017.07.06. Transl Lung Cancer Res. 2017. PMID: 29114473 Free PMC article. Review.
-
Assessing Genotype-Phenotype Correlations with Deep Learning in Colorectal Cancer: A Multi-Centric Study.medRxiv [Preprint]. 2025 Feb 8:2025.02.04.25321660. doi: 10.1101/2025.02.04.25321660. medRxiv. 2025. PMID: 39973981 Free PMC article. Preprint.
-
Immunohistochemical Study Using Monoclonal VE1 Antibody Can Substitute the Molecular Tests for Apprehension of BRAF V600E Mutation in Patients with Non-small-Cell Lung Carcinoma.Anal Cell Pathol (Amst). 2019 Nov 5;2019:2315673. doi: 10.1155/2019/2315673. eCollection 2019. Anal Cell Pathol (Amst). 2019. PMID: 31781475 Free PMC article.
References
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
-
- Capper D, Berghoff AS, Magerle M, et al. Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol. 2011;123:223–233. - PubMed
-
- Routhier CA, Mochel MC, Lynch K, et al. Comparison of 2 monoclonal antibodies for immunohistochemical detection of BRAF V600E mutation in malignant melanoma, pulmonary carcinoma, gastrointestinal carcinoma, thyroid carcinoma, and gliomas. Hum Pathol. 2013;44:2563–2570. - PubMed
-
- Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

