Establishment of highly tumorigenic human colorectal cancer cell line (CR4) with properties of putative cancer stem cells
- PMID: 24921652
- PMCID: PMC4055451
- DOI: 10.1371/journal.pone.0099091
Establishment of highly tumorigenic human colorectal cancer cell line (CR4) with properties of putative cancer stem cells
Abstract
Background: Colorectal cancer (CRC) has the third highest mortality rates among the US population. According to the most recent concept of carcinogenesis, human tumors are organized hierarchically, and the top of it is occupied by malignant stem cells (cancer stem cells, CSCs, or cancer-initiating cells, CICs), which possess unlimited self-renewal and tumor-initiating capacities and high resistance to conventional therapies. To reflect the complexity and diversity of human tumors and to provide clinically and physiologically relevant cancer models, large banks of characterized patient-derived low-passage cell lines, and especially CIC-enriched cell lines, are urgently needed.
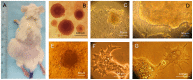
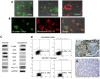
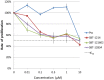
Principal findings: Here we report the establishment of a novel CIC-enriched, highly tumorigenic and clonogenic colon cancer cell line, CR4, derived from liver metastasis. This stable cell line was established by combining 3D culturing and 2D culturing in stem cell media, subcloning of cells with particular morphology, co-culture with carcinoma associated fibroblasts (CAFs) and serial transplantation to NOD/SCID mice. Using RNA-Seq complete transcriptome profiling of the tumorigenic fraction of the CR4 cells in comparison to the bulk tumor cells, we have identified about 360 differentially expressed transcripts, many of which represent stemness, pluripotency and resistance to treatment. Majority of the established CR4 cells express common markers of stemness, including CD133, CD44, CD166, EpCAM, CD24 and Lgr5. Using immunocytochemical, FACS and western blot analyses, we have shown that a significant ratio of the CR4 cells express key markers of pluripotency markers, including Sox-2, Oct3/4 and c-Myc. Constitutive overactivation of ABC transporters and NF-kB and absence of tumor suppressors p53 and p21 may partially explain exceptional drug resistance of the CR4 cells.
Conclusions: The highly tumorigenic and clonogenic CIC-enriched CR4 cell line may provide an important new tool to support the discovery of novel diagnostic and/or prognostic biomarkers as well as the development of more effective therapeutic strategies.
Conflict of interest statement
Figures




References
-
- Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62: 10–29. - PubMed
-
- Hutchinson L, Kirk R (2011) High drug attrition rates—where are we going wrong? Nat Rev Clin Oncol 8: 189–90. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105–11. - PubMed
-
- Clarke MF, Fuller M (2006) Stem cells and cancer: Two faces of eve. Cell 124: 1111–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

