Protein kinase C and extracellular signal-regulated kinase regulate movement, attachment, pairing and egg release in Schistosoma mansoni
- PMID: 24921927
- PMCID: PMC4055629
- DOI: 10.1371/journal.pntd.0002924
Protein kinase C and extracellular signal-regulated kinase regulate movement, attachment, pairing and egg release in Schistosoma mansoni
Abstract
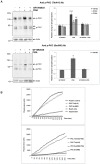
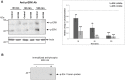
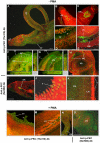
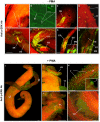
Protein kinases C (PKCs) and extracellular signal-regulated kinases (ERKs) are evolutionary conserved cell signalling enzymes that coordinate cell function. Here we have employed biochemical approaches using 'smart' antibodies and functional screening to unravel the importance of these enzymes to Schistosoma mansoni physiology. Various PKC and ERK isotypes were detected, and were differentially phosphorylated (activated) throughout the various S. mansoni life stages, suggesting isotype-specific roles and differences in signalling complexity during parasite development. Functional kinase mapping in adult worms revealed that activated PKC and ERK were particularly associated with the adult male tegument, musculature and oesophagus and occasionally with the oesophageal gland; other structures possessing detectable activated PKC and/or ERK included the Mehlis' gland, ootype, lumen of the vitellaria, seminal receptacle and excretory ducts. Pharmacological modulation of PKC and ERK activity in adult worms using GF109203X, U0126, or PMA, resulted in significant physiological disturbance commensurate with these proteins occupying a central position in signalling pathways associated with schistosome muscular activity, neuromuscular coordination, reproductive function, attachment and pairing. Increased activation of ERK and PKC was also detected in worms following praziquantel treatment, with increased signalling associated with the tegument and excretory system and activated ERK localizing to previously unseen structures, including the cephalic ganglia. These findings support roles for PKC and ERK in S. mansoni homeostasis, and identify these kinase groups as potential targets for chemotherapeutic treatments against human schistosomiasis, a neglected tropical disease of enormous public health significance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

