A high content clonogenic survival drug screen identifies mek inhibitors as potent radiation sensitizers for KRAS mutant non-small-cell lung cancer
- PMID: 24922006
- PMCID: PMC4110054
- DOI: 10.1097/JTO.0000000000000199
A high content clonogenic survival drug screen identifies mek inhibitors as potent radiation sensitizers for KRAS mutant non-small-cell lung cancer
Abstract
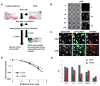
Introduction: Traditional clonogenic survival and high throughput colorimetric assays are inadequate as drug screens to identify novel radiation sensitizers. We developed a method that we call the high content clonogenic survival assay (HCSA) that will allow screening of drug libraries to identify candidate radiation sensitizers.
Methods: Drug screen using HCSA was done in 96 well plates. After drug treatment, irradiation, and incubation, colonies were stained with crystal violet and imaged on the INCell 6000 (GE Health). Colonies achieving 50 or more cells were enumerated using the INCell Developer image analysis software. A proof-of-principle screen was done on the KRAS mutant lung cancer cell line H460 and a Custom Clinical Collection (146 compounds).
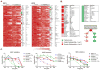
Results: Multiple drugs of the same class were found to be radiation sensitizers and levels of potency seemed to reflect the clinical relevance of these drugs. For instance, several PARP inhibitors were identified as good radiation sensitizers in the HCSA screen. However, there were also a few PARP inhibitors not found to be sensitizing that have either not made it into clinical development, or in the case of BSI-201, was proven to not even be a PARP inhibitor. We discovered that inhibitors of pathways downstream of activated mutant KRAS (PI3K, AKT, mTOR, and MEK1/2) sensitized H460 cells to radiation. Furthermore, the potent MEK1/2 inhibitor tramenitib selectively enhanced radiation effects in KRAS mutant but not wild-type lung cancer cells.
Conclusions: Drug screening for novel radiation sensitizers is feasible using the HCSA approach. This is an enabling technology that will help accelerate the discovery of novel radiosensitizers for clinical testing.
Conflict of interest statement
Figures
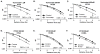

References
-
- Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nature Reviews Cancer. 2005;5:689–698. - PubMed
-
- Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. - PubMed
-
- Drew Y, Plummer R. PARP inhibitors in cancer therapy: Two modes of attack on the cancer cell widening the clinical applications. Drug Resistance Updates. 2009;12:153–156. - PubMed
-
- Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. The Lancet. 2010;376:235–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

