JNK controls the onset of mitosis in planarian stem cells and triggers apoptotic cell death required for regeneration and remodeling
- PMID: 24922054
- PMCID: PMC4055413
- DOI: 10.1371/journal.pgen.1004400
JNK controls the onset of mitosis in planarian stem cells and triggers apoptotic cell death required for regeneration and remodeling
Erratum in
-
Correction: JNK controls the onset of mitosis in planarian stem cells and triggers apoptotic cell death required for regeneration and remodeling.PLoS Genet. 2015 Apr 10;11(4):e1005132. doi: 10.1371/journal.pgen.1005132. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25860912 Free PMC article. No abstract available.
Abstract
Regeneration of lost tissues depends on the precise interpretation of molecular signals that control and coordinate the onset of proliferation, cellular differentiation and cell death. However, the nature of those molecular signals and the mechanisms that integrate the cellular responses remain largely unknown. The planarian flatworm is a unique model in which regeneration and tissue renewal can be comprehensively studied in vivo. The presence of a population of adult pluripotent stem cells combined with the ability to decode signaling after wounding enable planarians to regenerate a complete, correctly proportioned animal within a few days after any kind of amputation, and to adapt their size to nutritional changes without compromising functionality. Here, we demonstrate that the stress-activated c-jun-NH2-kinase (JNK) links wound-induced apoptosis to the stem cell response during planarian regeneration. We show that JNK modulates the expression of wound-related genes, triggers apoptosis and attenuates the onset of mitosis in stem cells specifically after tissue loss. Furthermore, in pre-existing body regions, JNK activity is required to establish a positive balance between cell death and stem cell proliferation to enable tissue renewal, remodeling and the maintenance of proportionality. During homeostatic degrowth, JNK RNAi blocks apoptosis, resulting in impaired organ remodeling and rescaling. Our findings indicate that JNK-dependent apoptotic cell death is crucial to coordinate tissue renewal and remodeling required to regenerate and to maintain a correctly proportioned animal. Hence, JNK might act as a hub, translating wound signals into apoptotic cell death, controlled stem cell proliferation and differentiation, all of which are required to coordinate regeneration and tissue renewal.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
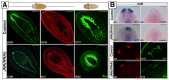
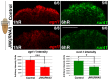
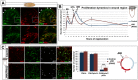
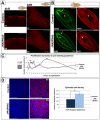




References
-
- Newmark PA, Sánchez Alvarado A (2002) Not your father's planarian: a classic model enters the era of functional genomics. Nat Rev Genet 3: 210–219. - PubMed
-
- Reddien PW, Sánchez Alvarado A (2004) Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol 20: 725–757. - PubMed
-
- Saló E (2006) The power of regeneration and the stem-cell kingdom: freshwater planarians (Platyhelminthes). Bioessays 28: 546–559. - PubMed
-
- Handberg-Thorsager M, Fernandez E, Saló E (2008) Stem cells and regeneration in planarians. Front Biosci 13: 6374–6394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

