Availability and use of molecular microbiological and immunological tests for the diagnosis of tuberculosis in europe
- PMID: 24922084
- PMCID: PMC4055680
- DOI: 10.1371/journal.pone.0099129
Availability and use of molecular microbiological and immunological tests for the diagnosis of tuberculosis in europe
Abstract
Introduction: Currently only limited data exist regarding the availability and clinical use of molecular and immunological tests for tuberculosis (TB) in the European setting.
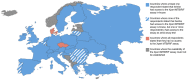
Methods: Web-based survey of Paediatric-Tuberculosis-Network-European-Trialsgroup (ptbnet) and Tuberculosis-Network-European-Trialsgroup (TBnet) members conducted June to December 2013. Both networks comprise clinicians, microbiologists, epidemiologists and researchers predominately based in Europe.
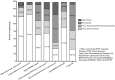
Results: 191 healthcare professionals from 31 European countries participated. Overall, 26.8% of respondents did not have access to the Xpert MTB/RIF assay; only 44.6% had access to the assay in-house. However, a substantial proportion had access to other commercial and/or non-commercial PCR-based assays for TB (68.8% and 31.8%, respectively). Only 6.4% did not have access to any PCR-based assays for TB. A large proportion of participants with access to the Xpert MTB/RIF assay had used it for the analysis of non-respiratory samples [pleural fluid: 36.5%, gastric aspirates: 34.7%, cerebrospinal fluid: 34.7%, stool samples: 4.3%, blood/serum: 2.6%, 'other samples' (which included biopsy/tissue samples, lymph node aspirates, joint aspirates and urine samples): 16.5%]. Regarding interferon-gamma release assays, a greater proportion of respondents had access to the QuantiFERON-TB Gold assay (84.7%) than to the T-SPOT.TB assay (52.2%).
Conclusions: Both immunological and molecular TB tests are widely available across Europe. The QuantiFERON-TB Gold assay is more widely used than the T-SPOT.TB assay, which may reflect the difficulties of integrating an ELISPOT assay into the routine laboratory setting. Although Xpert MTB/RIF assays are optimised and solely licensed for the analysis of sputum samples, in clinical practice they are commonly used for non-respiratory samples. Further research is needed to establish how current molecular TB tests impact on patient care and outcome in the routine clinical setting.
Conflict of interest statement
Figures
References
-
- Barnard M, Warren R, Gey Van Pittius N, van Helden P, Bosman M, et al. (2012) Genotype MTBDRsl line probe assay shortens time to diagnosis of extensively drug-resistant tuberculosis in a high-throughput diagnostic laboratory. Am J Respir Crit Care Med 186: 1298–1305. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical