Evolution and convergence of state laws governing controlled substance prescription monitoring programs, 1998-2011
- PMID: 24922132
- PMCID: PMC4103230
- DOI: 10.2105/AJPH.2014.301923
Evolution and convergence of state laws governing controlled substance prescription monitoring programs, 1998-2011
Abstract
Objectives: We sought to collect and characterize all laws governing the operation of prescription monitoring programs (PMPs), state-level databases that collect patient-specific prescription information, which have been suggested as a tool for reducing prescription drug overdose fatalities.
Methods: We utilized a structured legal research protocol to systematically identify, review, and code all PMP statutes and regulations effective from 1998 through 2011. These laws were then abstracted along eleven domains, including reporting provisions, data sharing, and data access.
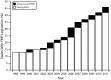
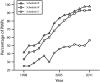
Results: PMP characteristics vary greatly among states and across time. We observed an increase in the types and frequency of data required to be reported, the types of individuals permitted to access PMP data, and the percentage of PMPs authorized to proactively identify outlier prescribers and patients. As of 2011, 10 states required PMPs to report suspicious activity to law enforcement, while only 3 required reporting to the patient's physician. None required linkage to drug treatment or required all prescribers to review PMP data before prescribing. Few explicitly address data retention.
Conclusions: State PMP laws are heterogeneous and evolving. Future studies of PMP effectiveness should take these variations into account.
Figures
Similar articles
-
Controlled Substance Prescribing Patterns--Prescription Behavior Surveillance System, Eight States, 2013.MMWR Surveill Summ. 2015 Oct 16;64(9):1-14. doi: 10.15585/mmwr.ss6409a1. MMWR Surveill Summ. 2015. PMID: 26469747
-
Prescribers and pharmacists requests for prescription monitoring program (PMP) data: does PMP structure matter?J Pain Palliat Care Pharmacother. 2013 Jun;27(2):136-42. doi: 10.3109/15360288.2013.788598. Epub 2013 May 20. J Pain Palliat Care Pharmacother. 2013. PMID: 23688495
-
Do more robust prescription drug monitoring programs reduce prescription opioid overdose?Addiction. 2017 Oct;112(10):1773-1783. doi: 10.1111/add.13741. Epub 2017 Feb 8. Addiction. 2017. PMID: 28009931
-
Effectiveness of Prescription Monitoring Programs in Reducing Opioid Prescribing, Dispensing, and Use Outcomes: A Systematic Review.J Pain. 2019 Dec;20(12):1383-1393. doi: 10.1016/j.jpain.2019.04.007. Epub 2019 May 3. J Pain. 2019. PMID: 31059823
-
Prescription Monitoring Programs for Optimizing Medication Use and Preventing Harm: A Review of Safety and Guidelines [Internet].Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019 Apr 23. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019 Apr 23. PMID: 31487132 Free Books & Documents. Review.
Cited by
-
A typology of prescription drug monitoring programs: a latent transition analysis of the evolution of programs from 1999 to 2016.Addiction. 2019 Feb;114(2):248-258. doi: 10.1111/add.14440. Epub 2018 Oct 22. Addiction. 2019. PMID: 30207015 Free PMC article.
-
Overdose Epidemic, Prescription Monitoring Programs, and Public Health: A Review of State Laws.Am J Public Health. 2015 Nov;105(11):e9-e11. doi: 10.2105/AJPH.2015.302856. Epub 2015 Sep 17. Am J Public Health. 2015. PMID: 26378849 Free PMC article.
-
Patterns of major depression and nonmedical use of prescription opioids in the United States.Drug Alcohol Depend. 2015 Aug 1;153:258-64. doi: 10.1016/j.drugalcdep.2015.05.010. Epub 2015 May 19. Drug Alcohol Depend. 2015. PMID: 26026492 Free PMC article.
-
Opioid Prescribing Laws Are Not Associated with Short-term Declines in Prescription Opioid Distribution.Pain Med. 2020 Mar 1;21(3):532-537. doi: 10.1093/pm/pnz159. Pain Med. 2020. PMID: 31365095 Free PMC article.
-
Association between cannabis laws and opioid prescriptions among privately insured adults in the US.Prev Med. 2019 Aug;125:62-68. doi: 10.1016/j.ypmed.2019.05.012. Epub 2019 May 21. Prev Med. 2019. PMID: 31125629 Free PMC article.
References
-
- Haythornthwaite JA, Menefee LA, Quatrano-Piacentini AL, Pappagallo M. Outcome of chronic opioid therapy for non-cancer pain. J Pain Symptom Manage. 1998;15(3):185–194. - PubMed
-
- Maier C, Hildebrandt J, Klinger R, Henrich-Eberl C, Lindena G, Group MS. Morphine responsiveness, efficacy and tolerability in patients with chronic non-tumor associated pain-results of a double-blind placebo-controlled trial (MONTAS) Pain. 2002;97(3):223–233. - PubMed
-
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349(20):1943–1953. - PubMed
-
- Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25(2):171–186. - PubMed
-
- Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

