Gd(DOTAlaP): exploring the boundaries of fast water exchange in gadolinium-based magnetic resonance imaging contrast agents
- PMID: 24922178
- PMCID: PMC4095929
- DOI: 10.1021/ic5008928
Gd(DOTAlaP): exploring the boundaries of fast water exchange in gadolinium-based magnetic resonance imaging contrast agents
Abstract
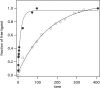
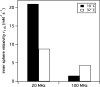
Here, we describe the synthesis of the single amino acid chelator DOTAlaP and four of its derivatives. The corresponding gadolinium(III) complexes were investigated for their kinetic inertness, relaxometric properties at a range of fields and temperatures, water exchange rate, and interaction with human serum albumin (HSA). Derivatives with one inner-sphere water (q = 1) were determined to have a mean water residency time between 8 and 6 ns in phoshate-buffered saline at 37 °C. The corresponding europium complexes were also formed and used to obtain information on the hydration number of the corresponding coordination complexes. Two complexes capable of binding HSA were also synthesized, of which one, Gd(5b), contains no inner-sphere water, while the other derivative, Gd(4b), is a mixture of ca. 15% q =1 and 85% q = 0. In the presence of HSA, the latter displayed a very short mean water residency time (τM(310) = 2.4 ns) and enhanced relaxivity at intermediate and high fields. The kinetic inertness of Gd(4b) with respect to complex dissociation was decreased compared to its DOTAla analogue but still 100-fold more inert than [Gd(BOPTA)(H2O)](2-). Magnetic resonance imaging in mice showed that Gd(4b) was able to provide 38% better vessel to muscle contrast compared to the clinically used HSA binding agent MS-325.
Figures





References
-
- Caravan P. Chem. Soc. Rev. 2006, 35, 512–523. - PubMed
-
- Chan K. W.-Y.; Wong W.-T. Coord. Chem. Rev. 2007, 251, 2428–2451.
-
- Helm L. Future Med. Chem. 2010, 2, 385–396. - PubMed
-
- Raymond K. N.; Pierre V. C. Bioconjugate Chem. 2005, 16, 3–8. - PubMed
-
- Caravan P.; Cloutier N. J.; Greenfield M. T.; McDermid S. A.; Dunham S. U.; Bulte J. W. M.; Amedio J. C.; Looby R. J.; Supkowski R. M.; Horrocks W. D.; McMurry T. J.; Lauffer R. B. J. Am. Chem. Soc. 2002, 124, 3152–3162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

