Platelet factor 4 protects bone marrow mesenchymal stem cells from acute radiation injury
- PMID: 24922360
- PMCID: PMC4112396
- DOI: 10.1259/bjr.20140184
Platelet factor 4 protects bone marrow mesenchymal stem cells from acute radiation injury
Abstract
Objective: The aim of this study was to find a new radiation protector, platelet factor 4 (PF4) and to identify its effect on haemopoietic microenvironment in vitro and in vivo.
Methods: Radiation damage on bone marrow mesenchymal stem cells ex and in vitro was set up as models. Growth curve analysis, clonogenic survival assay, FACSCalibur™ (BD Immunocytometry Systems, San Jose, CA), 5-ethynyl-2'-deoxyuridine immunofluorescence staining and quantitative reverse transcription-polymerase chain reaction were employed to assess the characterization of bone marrow mesenchymal stem cells (BMSCs), proliferation, apoptosis, cell cycle and gene expression.
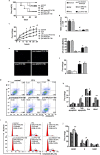
Results: A dose- and time-dependent enhancement of cell viability and survival was observed for PF4 treatment along with 500 cGy γ-radiation in vitro. The same phenomena were noted in vivo, including enhancement of adherence and proliferation ability while inhibition of cell apoptosis, which were associated with a short-term decrease in the G0/G1 ratio owing to S phase arrest. These were accompanied with enhanced Bcl-2 expression and p53/p21 loss.
Conclusion: These results uncover that PF4 might be a novel therapeutic approach, which could reduce DNA damage and increase survival of BMSCs, in part, by inhibiting p53/p21 axis and facilitating DNA damage repair.
Advances in knowledge: This study explores the feasibility of a new radioprotector and hence may be clinically important.
Figures



References
-
- Nicolay NH, Sommer E, Lopez R, Wirkner U, Trinh T, Sisombath S, et al. Mesenchymal stem cells retain their defining stem cell characteristics after exposure to ionizing radiation. Int J Radiat Oncol Biol Phys 2013; 87: 1171–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

