Muscle structure influences utrophin expression in mdx mice
- PMID: 24922526
- PMCID: PMC4055409
- DOI: 10.1371/journal.pgen.1004431
Muscle structure influences utrophin expression in mdx mice
Abstract
Duchenne muscular dystrophy (DMD) is a severe muscle wasting disorder caused by mutations in the dystrophin gene. To examine the influence of muscle structure on the pathogenesis of DMD we generated mdx4cv:desmin double knockout (dko) mice. The dko male mice died of apparent cardiorespiratory failure at a median age of 76 days compared to 609 days for the desmin-/- mice. An ∼ 2.5 fold increase in utrophin expression in the dko skeletal muscles prevented necrosis in ∼ 91% of 1a, 2a and 2d/x fiber-types. In contrast, utrophin expression was reduced in the extrasynaptic sarcolemma of the dko fast 2b fibers leading to increased membrane fragility and dystrophic pathology. Despite lacking extrasynaptic utrophin, the dko fast 2b fibers were less dystrophic than the mdx4cv fast 2b fibers suggesting utrophin-independent mechanisms were also contributing to the reduced dystrophic pathology. We found no overt change in the regenerative capacity of muscle stem cells when comparing the wild-type, desmin-/-, mdx4cv and dko gastrocnemius muscles injured with notexin. Utrophin could form costameric striations with α-sarcomeric actin in the dko to maintain the integrity of the membrane, but the lack of restoration of the NODS (nNOS, α-dystrobrevin 1 and 2, α1-syntrophin) complex and desmin coincided with profound changes to the sarcomere alignment in the diaphragm, deposition of collagen between the myofibers, and impaired diaphragm function. We conclude that the dko mice may provide new insights into the structural mechanisms that influence endogenous utrophin expression that are pertinent for developing a therapy for DMD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
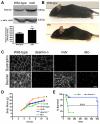
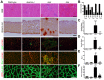

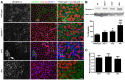





References
-
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, et al. (2012) Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 71: 304–313. - PubMed
-
- Hoffman EP, Brown RH Jr, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51: 919–928. - PubMed
-
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, et al. (1987) Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50: 509–517. - PubMed
-
- Bhasin N, Law R, Liao G, Safer D, Ellmer J, et al. (2005) Molecular extensibility of mini-dystrophins and a dystrophin rod construct. J Mol Biol 352: 795–806. - PubMed
-
- Mirza A, Sagathevan M, Sahni N, Choi L, Menhart N (2010) A biophysical map of the dystrophin rod. Biochim Biophys Acta 1804: 1796–1809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

