Clinical features and course of ocular toxocariasis in adults
- PMID: 24922534
- PMCID: PMC4055477
- DOI: 10.1371/journal.pntd.0002938
Clinical features and course of ocular toxocariasis in adults
Abstract
Purpose: To investigate the clinical features, clinical course of granuloma, serologic findings, treatment outcome, and probable infection sources in adult patients with ocular toxocariasis (OT).
Methods: In this retrospective cohort study, we examined 101 adult patients diagnosed clinically and serologically with OT. Serial fundus photographs and spectral domain optical coherence tomography images of all the patients were reviewed. A clinic-based case-control study on pet ownership, occupation, and raw meat ingestion history was performed to investigate the possible infection sources.
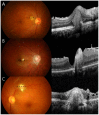
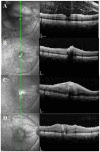
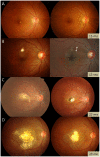
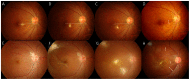
Results: Among the patients diagnosed clinically and serologically with OT, 69.6% showed elevated immunoglobulin E (IgE) levels. Granuloma in OT involved all retinal layers and several vitreoretinal comorbidities were noted depending on the location of granuloma: posterior pole granuloma was associated with epiretinal membrane and retinal nerve fiber layer defects, whereas peripheral granuloma was associated with vitreous opacity. Intraocular migration of granuloma was observed in 15 of 93 patients (16.1%). Treatment with albendazole (400 mg twice a day for 2 weeks) and corticosteroids (oral prednisolone; 0.5-1 mg/kg/day) resulted in comparable outcomes to patients on corticosteroid monotherapy; however, the 6-month recurrence rate in patients treated with combined therapy (17.4%) was significantly lower than that in patients treated with corticosteroid monotherapy (54.5%, P=0.045). Ingestion of raw cow liver (80.8%) or meat (71.2%) was significantly more common in OT patients than healthy controls.
Conclusions: Our study discusses the diagnosis, treatment, and prevention strategies for OT. Evaluation of total IgE, in addition to anti-toxocara antibody, can assist in the serologic diagnosis of OT. Combined albendazole and corticosteroid therapy may reduce intraocular inflammation and recurrence. Migrating feature of granuloma is clinically important and may further suggest the diagnosis of OT. Clinicians need to carefully examine comorbid conditions for OT. OT may be associated with ingestion of uncooked meat, especially raw cow liver, in adult patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU (2010) Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol 104: 3–23. - PubMed
-
- Stewart JM, Cubillan LD, Cunningham ET Jr (2005) Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina 25: 1005–1013. - PubMed
-
- Woodhall D, Starr MC, Montgomery SP, Jones JL, Lum F, et al. (2012) Ocular toxocariasis: epidemiologic, anatomic, and therapeutic variations based on a survey of ophthalmic subspecialists. Ophthalmology 119: 1211–1217. - PubMed
-
- Biglan AW, Glickman LT, Lobes LA Jr (1979) Serum and vitreous Toxocara antibody in nematode endophthalmitis. Am J Ophthalmol 88: 898–901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

