The classical lancefield antigen of group a Streptococcus is a virulence determinant with implications for vaccine design
- PMID: 24922575
- PMCID: PMC4078075
- DOI: 10.1016/j.chom.2014.05.009
The classical lancefield antigen of group a Streptococcus is a virulence determinant with implications for vaccine design
Abstract
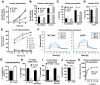
Group A Streptococcus (GAS) is a leading cause of infection-related mortality in humans. All GAS serotypes express the Lancefield group A carbohydrate (GAC), comprising a polyrhamnose backbone with an immunodominant N-acetylglucosamine (GlcNAc) side chain, which is the basis of rapid diagnostic tests. No biological function has been attributed to this conserved antigen. Here we identify and characterize the GAC biosynthesis genes, gacA through gacL. An isogenic mutant of the glycosyltransferase gacI, which is defective for GlcNAc side-chain addition, is attenuated for virulence in two infection models, in association with increased sensitivity to neutrophil killing, platelet-derived antimicrobials in serum, and the cathelicidin antimicrobial peptide LL-37. Antibodies to GAC lacking the GlcNAc side chain and containing only polyrhamnose promoted opsonophagocytic killing of multiple GAS serotypes and protected against systemic GAS challenge after passive immunization. Thus, the Lancefield antigen plays a functional role in GAS pathogenesis, and a deeper understanding of this unique polysaccharide has implications for vaccine development.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





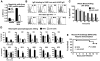
References
-
- Appleton RS, Victorica BE, Tamer D, Ayoub EM. Specificity of persistence of antibody to the streptococcal group A carbohydrate in rheumatic valvular heart disease. J Lab Clin Med. 1985;105:114–119. - PubMed
-
- Ayoub EM, Taranta A, Bartley TD. Effect of valvular surgery on antibody to the group A streptococcal carbohydrate. Circulation. 1974;50:144–150. - PubMed
-
- Bisno AL, Rubin FA, Cleary PP, Dale JB. Prospects for a group A streptococcal vaccine: rationale, feasibility, and obstacles--report of a National Institute of Allergy and Infectious Diseases workshop. Clin Infect Dis. 2005;41:1150–1156. - PubMed
-
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
