Cell-to-cell transfer of M. tuberculosis antigens optimizes CD4 T cell priming
- PMID: 24922576
- PMCID: PMC4098643
- DOI: 10.1016/j.chom.2014.05.007
Cell-to-cell transfer of M. tuberculosis antigens optimizes CD4 T cell priming
Abstract
During Mycobacterium tuberculosis and other respiratory infections, optimal T cell activation requires pathogen transport from the lung to a local draining lymph node (LN). However, the infected inflammatory monocyte-derived dendritic cells (DCs) that transport M. tuberculosis to the local lymph node are relatively inefficient at activating CD4 T cells, possibly due to bacterial inhibition of antigen presentation. We found that infected migratory DCs release M. tuberculosis antigens as soluble, unprocessed proteins for uptake and presentation by uninfected resident lymph node DCs. This transfer of bacterial proteins from migratory to local DCs results in optimal priming of antigen-specific CD4 T cells, which are essential in controlling tuberculosis. Additionally, this mechanism does not involve transfer of the whole bacterium and is distinct from apoptosis or exosome shedding. These findings reveal a mechanism that bypasses pathogen inhibition of antigen presentation by infected cells and generates CD4 T cell responses that control the infection.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
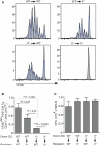
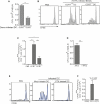




Comment in
-
Antigen smuggling in tuberculosis.Cell Host Microbe. 2014 Jun 11;15(6):657-9. doi: 10.1016/j.chom.2014.05.018. Cell Host Microbe. 2014. PMID: 24922567
References
-
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. - PubMed
-
- Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. - PubMed
-
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

