Discovery and structure-activity relationship of novel 2,3-dihydrobenzofuran-7-carboxamide and 2,3-dihydrobenzofuran-3(2H)-one-7-carboxamide derivatives as poly(ADP-ribose)polymerase-1 inhibitors
- PMID: 24922587
- PMCID: PMC4094269
- DOI: 10.1021/jm5002502
Discovery and structure-activity relationship of novel 2,3-dihydrobenzofuran-7-carboxamide and 2,3-dihydrobenzofuran-3(2H)-one-7-carboxamide derivatives as poly(ADP-ribose)polymerase-1 inhibitors
Abstract
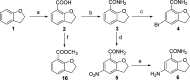
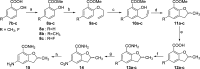
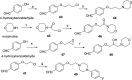
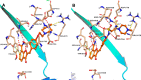
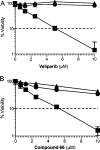
Novel substituted 2,3-dihydrobenzofuran-7-carboxamide (DHBF-7-carboxamide) and 2,3-dihydrobenzofuran-3(2H)-one-7-carboxamide (DHBF-3-one-7-carboxamide) derivatives were synthesized and evaluated as inhibitors of poly(ADP-ribose)polymerase-1 (PARP-1). A structure-based design strategy resulted in lead compound 3 (DHBF-7-carboxamide; IC50 = 9.45 μM). To facilitate synthetically feasible derivatives, an alternative core was designed, DHBF-3-one-7-carboxamide (36, IC50 = 16.2 μM). The electrophilic 2-position of this scaffold was accessible for extended modifications. Substituted benzylidene derivatives at the 2-position were found to be the most potent, with 3',4'-dihydroxybenzylidene 58 (IC50 = 0.531 μM) showing a 30-fold improvement in potency. Various heterocycles attached at the 4'-hydroxyl/4'-amino of the benzylidene moiety resulted in significant improvement in inhibition of PARP-1 activity (e.g., compounds 66-68, 70, 72, and 73; IC50 values from 0.718 to 0.079 μM). Compound 66 showed selective cytotoxicity in BRCA2-deficient DT40 cells. Crystal structures of three inhibitors (compounds (-)-13c, 59, and 65) bound to a multidomain PARP-1 structure were obtained, providing insights into further development of these inhibitors.
Figures








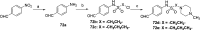

References
-
- Gibson B. A.; Kraus W. L. New insights into the molecular and cellular functions of poly(ADPribose) and PARPs. Nat. Rev. Mol. Cell Biol. 2012, 13, 411–424. - PubMed
-
- Hottiger M. O.; Hassa P. O.; Luscher B.; Schuler H.; Koch-Nolte F. Toward a unified nomenclature for mammalian ADP ribosyl transferases. Trends Biochem. Sci. 2010, 35, 208–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

