Impact of iron overload and potential benefit from iron chelation in low-risk myelodysplastic syndrome
- PMID: 24923296
- PMCID: PMC4467862
- DOI: 10.1182/blood-2014-03-563221
Impact of iron overload and potential benefit from iron chelation in low-risk myelodysplastic syndrome
Abstract
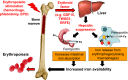
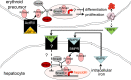
Myelodysplastic syndromes (MDSs) are a group of heterogeneous clonal bone marrow disorders characterized by ineffective hematopoiesis, peripheral blood cytopenias, and potential for malignant transformation. Lower/intermediate-risk MDSs are associated with longer survival and high red blood cell (RBC) transfusion requirements resulting in secondary iron overload. Recent data suggest that markers of iron overload portend a relatively poor prognosis, and retrospective analysis demonstrates that iron chelation therapy is associated with prolonged survival in transfusion-dependent MDS patients. New data provide concrete evidence of iron's adverse effects on erythroid precursors in vitro and in vivo. Renewed interest in the iron field was heralded by the discovery of hepcidin, the main serum peptide hormone negative regulator of body iron. Evidence from β-thalassemia suggests that regulation of hepcidin by erythropoiesis dominates regulation by iron. Because iron overload develops in some MDS patients who do not require RBC transfusions, the suppressive effect of ineffective erythropoiesis on hepcidin may also play a role in iron overload. We anticipate that additional novel tools for measuring iron overload and a molecular-mechanism-driven description of MDS subtypes will provide a deeper understanding of how iron metabolism and erythropoiesis intersect in MDSs and improve clinical management of this patient population.
© 2014 by The American Society of Hematology.
Figures

References
-
- Hellström-Lindberg E. Management of anemia associated with myelodysplastic syndrome. Semin Hematol. 2005;42(2 suppl 1):S10–S13. - PubMed
-
- Greenberg PL, Young NS, Gattermann N. Myelodysplastic syndromes. Hematology (Am Soc Hematol Educ Program) 2002:136–161. - PubMed
-
- Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503–3510. - PubMed
-
- Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594–7603. - PubMed
-
- Delaby C, Pilard N, Puy H, Canonne-Hergaux F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: early mRNA induction by haem, followed by iron-dependent protein expression. Biochem J. 2008;411(1):123–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

