Kisspeptin regulation of genes involved in cell invasion and angiogenesis in first trimester human trophoblast cells
- PMID: 24923321
- PMCID: PMC4055688
- DOI: 10.1371/journal.pone.0099680
Kisspeptin regulation of genes involved in cell invasion and angiogenesis in first trimester human trophoblast cells
Abstract
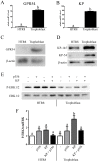
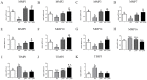
The precise regulation of extravillous trophoblast invasion of the uterine wall is a key process in successful pregnancies. Kisspeptin (KP) has been shown to inhibit cancer cell metastasis and placental trophoblast cell migration. In this study primary cultures of first trimester human trophoblast cells have been utilized in order to study the regulation of invasion and angiogenesis-related genes by KP. Trophoblast cells were isolated from first trimester placenta and their identity was confirmed by immunostaining for cytokeratin-7. Real-time quantitative RT-PCR demonstrated that primary trophoblast cells express higher levels of GPR54 (KP receptor) and KP mRNA than the trophoblast cell line HTR8Svneo. Furthermore, trophoblast cells also expressed higher GPR54 and KP protein levels. Treating primary trophoblast cells with KP induced ERK1/2 phosphorylation, while co-treating the cells with a KP antagonist almost completely blocked the activation of ERK1/2 and demonstrated that KP through its cognate GPR54 receptor can activate ERK1/2 in trophoblast cells. KP reduced the migratory capability of trophoblast cells in a scratch-migration assay. Real-time quantitative RT-PCR demonstrated that KP treatment reduced the expression of matrix metalloproteinase 1, 2, 3, 7, 9, 10, 14 and VEGF-A, and increased the expression of tissue inhibitors of metalloproteinases 1 and 3. These results suggest that KP can inhibit first trimester trophoblast cells invasion via inhibition of cell migration and down regulation of the metalloproteinase system and VEGF-A.
Conflict of interest statement
Figures



References
-
- Pijnenborg R, Bland JM, Robertson WB, Brosens I (1983) Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 4: 397–413. - PubMed
-
- Khong TY, De Wolf F, Robertson WB, Brosens I (1986) Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 93: 1049–1059. - PubMed
-
- Tseng JJ, Chou MM, Hsieh YT, Wen MC, Ho ES, et al. (2006) Differential expression of vascular endothelial growth factor, placenta growth factor and their receptors in placentae from pregnancies complicated by placenta accreta. Placenta 27: 70–78. - PubMed
-
- Hiden U, Bilban M, Knofler M, Desoye G (2007) Kisspeptins and the placenta: regulation of trophoblast invasion. Rev Endocr Metab Disord 8: 31–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

