A method to identify and validate mitochondrial modulators using mammalian cells and the worm C. elegans
- PMID: 24923838
- PMCID: PMC4055904
- DOI: 10.1038/srep05285
A method to identify and validate mitochondrial modulators using mammalian cells and the worm C. elegans
Abstract
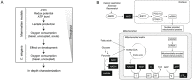
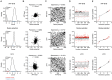
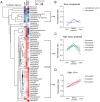
Mitochondria are semi-autonomous organelles regulated by a complex network of proteins that are vital for many cellular functions. Because mitochondrial modulators can impact many aspects of cellular homeostasis, their identification and validation has proven challenging. It requires the measurement of multiple parameters in parallel to understand the exact nature of the changes induced by such compounds. We developed a platform of assays scoring for mitochondrial function in two complementary models systems, mammalian cells and C. elegans. We first optimized cell culture conditions and established the mitochondrial signature of 1,200 FDA-approved drugs in liver cells. Using cell-based and C. elegans assays, we further defined the metabolic effects of two pharmacological classes that emerged from our hit list, i.e. imidazoles and statins. We found that these two drug classes affect respiration through different and cholesterol-independent mechanisms in both models. Our screening strategy enabled us to unequivocally identify compounds that have toxic or beneficial effects on mitochondrial activity. Furthermore, the cross-species approach provided novel mechanistic insight and allowed early validation of hits that act on mitochondrial function.
Conflict of interest statement
J.A. is a co-founder and scientific advisor to Mitokyne. None of the other authors declare a conflict of interest.
Figures




References
-
- Wallace D. C. Mitochondrial diseases in man and mouse. Science. 283, 1482–8 (1999). - PubMed
-
- Vafai S. B. & Mootha V. K. Mitochondrial disorders as windows into an ancient organelle. Nature. 491, 374–83 (2012). - PubMed
-
- Argmann C. A., Chambon P. & Auwerx J. Mouse phenogenomics: the fast track to “systems metabolism”. Cell Metab. 2, 349–60 (2005). - PubMed
-
- Peters L. L. et al. The mouse as a model for human biology: a resource guide for complex trait analysis. Nat Rev Genet. 8, 58–69 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

