Caffeic acid phenethyl amide ameliorates ischemia/reperfusion injury and cardiac dysfunction in streptozotocin-induced diabetic rats
- PMID: 24923878
- PMCID: PMC4065079
- DOI: 10.1186/1475-2840-13-98
Caffeic acid phenethyl amide ameliorates ischemia/reperfusion injury and cardiac dysfunction in streptozotocin-induced diabetic rats
Abstract
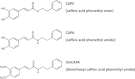
Background: Caffeic acid phenethyl ester (CAPE) has been shown to protect the heart against ischemia/reperfusion (I/R) injury by various mechanisms including its antioxidant effect. In this study, we evaluated the protective effects of a CAPE analog with more structural stability in plasma, caffeic acid phenethyl amide (CAPA), on I/R injury in streptozotocin (STZ)-induced type 1 diabetic rats.
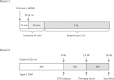
Methods: Type 1 diabetes mellitus was induced in Sprague-Dawley rats by a single intravenous injection of 60 mg/kg STZ. To produce the I/R injury, the left anterior descending coronary artery was occluded for 45 minutes, followed by 2 hours of reperfusion. CAPA was pretreated intraperitoneally 30 minutes before reperfusion. An analog devoid of the antioxidant property of CAPA, dimethoxyl CAPA (dmCAPA), and a nitric oxide synthase (NOS) inhibitor (Nω-nitro-l-arginine methyl ester [l-NAME]) were used to evaluate the mechanism involved in the reduction of the infarct size following CAPA-treatment. Finally, the cardioprotective effect of chronic treatment of CAPA was analyzed in diabetic rats.
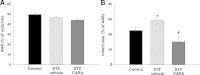
Results: Compared to the control group, CAPA administration (3 and 15 mg/kg) significantly reduced the myocardial infarct size after I/R, while dmCAPA (15 mg/kg) had no cardioprotective effect. Interestingly, pretreatment with a NOS inhibitor, (L-NAME, 3 mg/kg) eliminated the effect of CAPA on myocardial infarction. Additionally, a 4-week CAPA treatment (1 mg/kg, orally, once daily) started 4 weeks after STZ-induction could effectively decrease the infarct size and ameliorate the cardiac dysfunction by pressure-volume loop analysis in STZ-induced diabetic animals.
Conclusions: CAPA, which is structurally similar to CAPE, exerts cardioprotective activity in I/R injury through its antioxidant property and by preserving nitric oxide levels. On the other hand, chronic CAPA treatment could also ameliorate cardiac dysfunction in diabetic animals.
Figures




Similar articles
-
Caffeic acid phenethyl amide improves glucose homeostasis and attenuates the progression of vascular dysfunction in Streptozotocin-induced diabetic rats.Cardiovasc Diabetol. 2013 Jul 6;12:99. doi: 10.1186/1475-2840-12-99. Cardiovasc Diabetol. 2013. PMID: 23829275 Free PMC article.
-
Cardioprotection of CAPE-oNO2 against myocardial ischemia/reperfusion induced ROS generation via regulating the SIRT1/eNOS/NF-κB pathway in vivo and in vitro.Redox Biol. 2018 May;15:62-73. doi: 10.1016/j.redox.2017.11.023. Epub 2017 Nov 29. Redox Biol. 2018. PMID: 29220696 Free PMC article.
-
Inhibition of Rho kinase protects from ischaemia-reperfusion injury via regulation of arginase activity and nitric oxide synthase in type 1 diabetes.Diab Vasc Dis Res. 2017 May;14(3):236-245. doi: 10.1177/1479164116687935. Epub 2017 Feb 9. Diab Vasc Dis Res. 2017. PMID: 28183205
-
Effect of Ischemic Postconditioning on Myocardial Function and Infarct Size Following Reperfusion Injury in Diabetic Rats Pretreated With Vildagliptin.J Cardiovasc Pharmacol Ther. 2018 Mar;23(2):174-183. doi: 10.1177/1074248417729881. Epub 2017 Sep 13. J Cardiovasc Pharmacol Ther. 2018. PMID: 28901167
-
Cardiovascular Effects of Caffeic Acid and Its Derivatives: A Comprehensive Review.Front Physiol. 2020 Nov 27;11:595516. doi: 10.3389/fphys.2020.595516. eCollection 2020. Front Physiol. 2020. PMID: 33343392 Free PMC article. Review.
Cited by
-
Effect of photobiomodulation therapy on oxidative stress markers of gastrocnemius muscle of diabetic rats subjected to high-intensity exercise.Lasers Med Sci. 2018 Nov;33(8):1781-1790. doi: 10.1007/s10103-018-2540-7. Epub 2018 May 28. Lasers Med Sci. 2018. PMID: 29808322
-
SIRT1 protects against myocardial ischemia-reperfusion injury via activating eNOS in diabetic rats.Cardiovasc Diabetol. 2015 Oct 21;14:143. doi: 10.1186/s12933-015-0299-8. Cardiovasc Diabetol. 2015. PMID: 26489513 Free PMC article.
-
N-Propargyl caffeate amide (PACA) prevents cardiac fibrosis in experimental myocardial infarction by promoting pro-resolving macrophage polarization.Aging (Albany NY). 2020 Mar 23;12(6):5384-5398. doi: 10.18632/aging.102959. Epub 2020 Mar 23. Aging (Albany NY). 2020. PMID: 32203054 Free PMC article.
-
Modulatory effect of caffeic acid in alleviating diabetes and associated complications.World J Diabetes. 2023 Feb 15;14(2):62-75. doi: 10.4239/wjd.v14.i2.62. World J Diabetes. 2023. PMID: 36926656 Free PMC article. Review.
-
Therapeutic effects of pentoxifylline on diabetic heart tissue via NOS.Anatol J Cardiol. 2016 May;16(5):310-5. doi: 10.5152/akd.2015.6252. Epub 2015 May 5. Anatol J Cardiol. 2016. PMID: 26488377 Free PMC article.
References
-
- Di Filippo C, Marfella R, Cuzzocrea S, Piegari E, Petronella P, Giugliano D, Rossi F, D'Amico M. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes. 2005;54:803–810. doi: 10.2337/diabetes.54.3.803. - DOI - PubMed
-
- Marfella R, D'Amico M, Di Filippo C, Piegari E, Nappo F, Esposito K, Berrino L, Rossi F, Giugliano D. Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia. 2002;45:1172–1181. doi: 10.1007/s00125-002-0882-x. - DOI - PubMed
-
- Simpson PJ, Lucchesi BR. Free radicals and myocardial ischemia and reperfusion injury. J Lab Clin Med. 1987;110:13–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

