Pattern of mutation rates in the germline of Drosophila melanogaster males from a large-scale mutation screening experiment
- PMID: 24924332
- PMCID: PMC4132180
- DOI: 10.1534/g3.114.011056
Pattern of mutation rates in the germline of Drosophila melanogaster males from a large-scale mutation screening experiment
Abstract
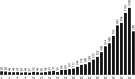
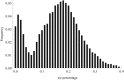
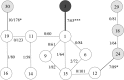
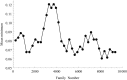
The sperm or eggs of sexual organisms go through a series of cell divisions from the fertilized egg; mutations can occur at each division. Mutations in the lineage of cells leading to the sperm or eggs are of particular importance because many such mutations may be shared by somatic tissues and also may be inherited, thus having a lasting consequence. For decades, little has been known about the pattern of the mutation rates along the germline development. Recently it was shown from a small portion of data that resulted from a large-scale mutation screening experiment that the rates of recessive lethal or nearly lethal mutations differ dramatically during the germline development of Drosophila melanogaster males. In this paper the full data set from the experiment and its analysis are reported by taking advantage of a recent methodologic advance. By analyzing the mutation patterns with different levels of recessive lethality, earlier published conclusions based on partial data are found to remain valid. Furthermore, it is found that for most nearly lethal mutations, the mutation rate at the first cell division is even greater than previous thought compared with those at other divisions. There is also some evidence that the mutation rate at the second division decreases rapidly but is still appreciably greater than those for the rest of the cleavage stage. The mutation rate at spermatogenesis is greater than late cleavage and stem-cell stages, but there is no evidence that rates are different among the five cell divisions of the spermatogenesis. We also found that a modestly biased sampling, leading to slightly more primordial germ cells after the eighth division than those reported in the literature, provides the best fit to the data. These findings provide conceptual and numerical basis for exploring the consequences of differential mutation rates during individual development.
Keywords: Drosophila melanogaster; cell coalescent; germline mutation rate; likelihood inference.
Copyright © 2014 Gao et al.
Figures

References
-
- Abrahamson S., Lewis E. B., 1971. The detection of mutations in Drosophila melanogaster, pp. 461–484 in Chemical Mutagens, edited by Hollaender A. Plenum Press, New York
-
- Ashburner M., 1989. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
-
- Brodberg R. K., Mitchell M. J., Smith S. L., Woodruff R. C., 1987. Specific reduction of N-dimethylnitro-samine mutagenicity in Drosophila melanogaster by dimethyl sulfoxide. Environ. Mol. Mutagen. 10: 425–432 - PubMed
-
- Crow J. F., 2006. Age and sex effects on human mutation rates: an old problem with new complexities. J. Radiat. Res. 47 Suppl B: B75–82 - PubMed
-
- Drost J. B., Lee W. R., 1995. Biological basis of germline mutation: Comparisons of spontaneous germline mutation rates among Drosophila, mouse and human. Environ. Mol. Mutagen. 25(Suppl 26): 48–64 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
