Oral misoprostol for induction of labour
- PMID: 24924489
- PMCID: PMC6513439
- DOI: 10.1002/14651858.CD001338.pub3
Oral misoprostol for induction of labour
Abstract
Background: Misoprostol is an orally active prostaglandin. In most countries misoprostol is not licensed for labour induction, but its use is common because it is cheap and heat stable.
Objectives: To assess the use of oral misoprostol for labour induction in women with a viable fetus.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (17 January 2014).
Selection criteria: Randomised trials comparing oral misoprostol versus placebo or other methods, given to women with a viable fetus for labour induction.
Data collection and analysis: Two review authors independently assessed trial data, using centrally-designed data sheets.
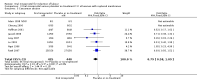
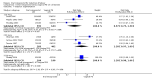
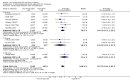
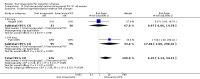
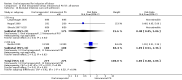
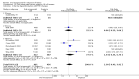
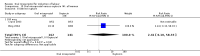
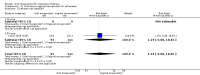
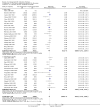
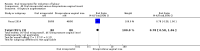
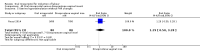
Main results: Overall there were 76 trials (14,412) women) which were of mixed quality.In nine trials comparing oral misoprostol with placebo (1109 women), women using oral misoprostol were more likely to give birth vaginally within 24 hours (risk ratio (RR) 0.16, 95% confidence interval (CI) 0.05 to 0.49; one trial; 96 women), need less oxytocin (RR 0.42, 95% CI 0.37 to 0.49; seven trials; 933 women) and have a lower caesarean section rate (RR 0.72, 95% CI 0.54 to 0.95; eight trials; 1029 women).In 12 trials comparing oral misoprostol with vaginal dinoprostone (3859 women), women given oral misoprostol were less likely to need a caesarean section (RR 0.88, 95% CI 0.78 to 0.99; 11 trials; 3592 women). There was some evidence that they had slower inductions, but there were no other statistically significant differences.Nine trials (1282 women) compared oral misoprostol with intravenous oxytocin. The caesarean section rate was significantly lower in women who received oral misoprostol (RR 0.77, 95% CI 0.60 to 0.98; nine trials; 1282 women), but they had increased rates of meconium-stained liquor (RR 1.65, 95% CI 1.04 to 2.60; seven trials; 1172 women).Thirty-seven trials (6417 women) compared oral and vaginal misoprostol and found no statistically significant difference in the primary outcomes of serious neonatal morbidity/death or serious maternal morbidity or death. The results for vaginal birth not achieved in 24 hours, uterine hyperstimulation with fetal heart rate (FHR) changes, and caesarean section were highly heterogenous - for uterine hyperstimulation with FHR changes this was related to dosage with lower rates in those with lower doses of oral misoprostol. However, there were fewer babies born with a low Apgar score in the oral group (RR 0.60, 95% CI 0.44 to 0.82; 19 trials; 4009 babies) and a decrease in postpartum haemorrhage (RR 0.57, 95% CI 0.34 to 0.95; 10 trials; 1478 women). However, the oral misoprostol group had an increase in meconium-stained liquor (RR 1.22, 95% CI 1.03 to 1.44; 24 trials; 3634 women).
Authors' conclusions: Oral misoprostol as an induction agent is effective at achieving vaginal birth. It is more effective than placebo, as effective as vaginal misoprostol and results in fewer caesarean sections than vaginal dinoprostone or oxytocin.Where misoprostol remains unlicensed for the induction of labour, many practitioners will prefer to use a licensed product like dinoprostone. If using oral misoprostol, the evidence suggests that the dose should be 20 to 25 mcg in solution. Given that safety is the primary concern, the evidence supports the use of oral regimens over vaginal regimens. This is especially important in situations where the risk of ascending infection is high and the lack of staff means that women cannot be intensely monitored.
Conflict of interest statement
Zarko Alfirevic is one of the principal investigators of a trial included in this review. He acted as an adviser and co‐investigator on the Phase III trials to companies involved in the development of misoprostol products for labour induction, but payments were on a one‐off basis with no regular or long‐lasting personal relationships with any organisation. Neither he, nor his immediate family, hold any shares or stocks in any company. Andrew Weeks runs the
Figures


































































































































































































































































Update of
-
Oral misoprostol for induction of labour.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD001338. doi: 10.1002/14651858.CD001338.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2014 Jun 13;(6):CD001338. doi: 10.1002/14651858.CD001338.pub3. PMID: 16625542 Updated.
Comment in
-
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS (CDSR) ISSUE 2-4, 2016.J Midwifery Womens Health. 2016 Jul;61(4):513-5. doi: 10.1111/jmwh.12501_2. J Midwifery Womens Health. 2016. PMID: 27462756 No abstract available.
References
References to studies included in this review
Aalami‐Harandi 2013 (T) {published data only}
-
- Aalami‐Harandi R, Karamali M, Moeini A. Induction of labor with titrated oral misoprostol solution versus oxytocin in term pregnancy: randomized controlled trial. Revista Brasileira de Ginecologia e Obstetricia 2013;35(2):60‐5. - PubMed
Adair 1998 (V50) {published data only}
-
- Adair CD, Weeks JW, Barrilleaux PS, Philibert L, Edwards MS, Lewis DF. Labor induction with oral versus vaginal misoprostol: a randomized, double‐blind trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S93. - PubMed
-
- Adair CD, Weeks JW, Barrilleaux S, Edwards M, Burlison K, Lewis DF. Oral or vaginal misoprostol administration for induction of labor: a randomised, double‐blind trial. Obstetrics & Gynecology 1998;92(5):810‐3. - PubMed
Adam 2005 (V50) {published data only}
-
- Adam I, Hassan OA, Elhassan EM. Oral misoprostol vs. vaginal misoprostol for cervical ripening and labour induction. International Journal of Gynecology & Obstetrics 2005;89:142‐3. - PubMed
Al‐Hussaini 2003 {published data only}
-
- Al‐Hussaini TK, Abdel‐Aal SA, Youssef MA. Oral misoprostol vs. intravenous oxytocin for labor induction in women with prelabor rupture of membranes at term. International Journal of Gynecology & Obstetrics 2003;82(1):73‐5. - PubMed
Bartha 2000 {published data only}
-
- Bartha JL, Comino‐Delgado R, Garcia‐Benasach F, Martinez‐Del‐Fresno P, Moreno‐Corral LJ. Oral misoprostol and intracervical dinoprostone for cervical ripening and labor induction: a randomized comparison. Obstetrics & Gynecology 2000;96:465‐9. - PubMed
Beigi 2003 {published data only}
-
- Beigi A, Kabiri M, Zarrinkoub F. Cervical ripening with oral misoprostol at term. International Journal of Gynecology & Obstetrics 2003;83:251‐5. - PubMed
Bennett 1998 (V50) {published data only}
-
- Bennett K, Butt K, Crane J, Hutchens D, Young D. Misoprostol for labour induction at term. Society of Obstetricians and Gynaecologists of Canada 54th Annual Meeting; 1998 June; Victoria, Canada. 1998:11.
-
- Bennett KA, Butt K, Crane JMG, Hutchens D, Young DC. A masked randomized comparison of oral and vaginal administration of misoprostol for labor induction. Obstetrics & Gynecology 1998;92(4 Suppl 1):481‐6. - PubMed
Butt 1999 {published data only}
-
- Butt KD, Bennett KA, Crane JM, Hutchens D, Young DC. Randomized comparison of oral misoprostol and oxytocin for labor induction in term prelabor membrane rupture. Obstetrics & Gynecology 1999;94(6):994‐9. - PubMed
Carlan 2001 (V50) {published data only}
-
- Carlan SJ, Bouldin S, Blust D, O'Brien WF. Safety and efficacy of misoprostol orally and vaginally: a randomized trial. Obstetrics & Gynecology 2001;98:107‐12. - PubMed
Cheng 2008 (T) (V25) {published data only}
-
- Cheng SY, Ming H, Lee JC. Titrated oral compared with vaginal misoprostol for labor induction: a randomized controlled trial. Obstetrics & Gynecology 2008;111(1):119‐25. - PubMed
Cheung 2006 {published data only}
-
- Cheung PC, Yeo EL, Wong KS, Tang LC. Oral misoprostol for induction of labor in prelabor rupture of membranes (PROM) at term: a randomized control trial. Acta Obstetricia et Gynecologica Scandinavica 2006;85(9):1128‐33. - PubMed
Colon 2005 (V25) {published data only}
-
- Colon I, Clawson K, Hunter K, Druzin ML, Taslimi MM. Prospective randomized clinical trial of inpatient cervical ripening with stepwise oral misoprostol vs vaginal misoprostol. American Journal of Obstetrics and Gynecology 2005;192:747‐52. - PubMed
-
- Colon I, Clawson K, Taslimi M, Druzin M. Prospective randomized clinical trial of inpatient cervical ripening with stepwise oral misoprostol. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S15. - PubMed
Crane 2003 {published data only}
-
- Crane J, Delaney T, Hutchens D. Oral misoprostol labor induction in term prelabor membrane rupture. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S168. - PubMed
-
- Crane JMG, Delaney T, Hutchens D. Oral misoprostol for premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 2003;189:720‐4. - PubMed
Dallenbach 2003 (T) {published data only}
-
- Dallenbach P, Boulvain M, Viardot C, Irion O. Oral misoprostol or vaginal dinoprostone for labor induction: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2003;188(1):162‐7. - PubMed
-
- Dallenbach P, Boulvain M, Viardot C, Irion O. Oral misoprostol or vaginal dinoprostone for labor induction? A randomized controlled trial [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S108. - PubMed
De 2006 {published data only}
-
- De A, Bagga R, Gopalan S. The routine use of oxytocin after oral misoprostol for labour induction in women with an unfavourable cervix is not of benefit. Australian and New Zealand Journal of Obstetrics and Gynaecology 2006;46(4):323‐9. - PubMed
Deshmukh 2013 (V50) {published data only}
Dodd 2006 {published data only}
-
- Dodd J, Crowther C, Ronbinson J. Misoprostol for the induction of labour at term: a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2005;45:347‐8.
-
- Dodd JM, Crowther CA, Robinson JS. Factors associated with adverse maternal health outcomes following induction of labour at term: analyses from a randomised trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:86.
-
- Dodd JM, Crowther CA, Robinson JS. Time of commencing induction of labour: a nested randomised controlled trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:85.
Dodd 2006a (T) {published data only}
-
- Dodd JM, Crowther CA, Robinson JS. Oral misoprostol versus intravenous oxytocin for induction of labour following artificial or spontaneous rupture of membranes: a randomised controlled trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:258.
Dyar 2000 (V50) {published data only}
-
- Dyar TR, Greig P, Cummings R, Nichols K. The efficacy and safety of oral versus vaginal misoprostol for the induction of term labor. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S135.
Elhassan 2007 (V50) {published data only}
-
- Elhassan EM, Nasr AM, Adam I. Sublingual compared with oral and vaginal misoprostol for labor induction. International Journal of Gynecology & Obstetrics 2007;97(2):153‐4. - PubMed
Fisher 2001 (V50) {published data only}
-
- Fisher S, Davies G, Mackenzie P. Oral versus vaginal misoprostol for induction of labour: a double‐blind, placebo‐controlled randomised trial. American Journal of Obstetrics and Gynecology 2001;184(1):S117. - PubMed
-
- Fisher S, Mackenzie V, Davies G. Oral versus vaginal misoprostol for induction of labor: a double‐blind randomized controlled trial. American Journal of Obstetrics and Gynecology 2001;185:906‐10. - PubMed
Gherman 2001 {published data only}
-
- Browning J, Gherman RB. Oral misoprostol versus intravaginal prostaglandin E2 for preinduction cervical ripening: a randomized trial. Obstetrics & Gynecology 2000;95(4 Suppl):76S. - PubMed
-
- Gherman R, Browning J, O'Boyle A, Murphy Goodwin T. Oral misoprostol vs. intravaginal prostaglandin e2 for preinduction cervical ripening. Journal of Reproductive Medicine 2001;46(7):641‐6. - PubMed
Hall 2002 (V25) {published data only}
-
- Hall R, Duarte‐Gardea M, Harlass F. Oral versus vaginal misoprostol for labor induction. Obstetrics & Gynecology 2002;99(6):1044‐8. - PubMed
Henrich 2008 (T) {published data only}
-
- Henrich W, Dudenhausen JW, Hanel C, Chen FC. Oral misoprostol against vaginal dinoprostone for labor induction at term: a randomized comparison [Prospektiv randomisierte Studie zum Vergleich von Misoprostol oral mit Dinoproston vaginal bei der Geburtseinleitung am Termin]. Zeitschrift fur Geburtshilfe und Neonatologie 2008;212(5):183‐8. - PubMed
Hoffman 2001 {published data only}
-
- Hoffman R, Anthony J, Fawcus J. Oral misoprostol vs. placebo in the management of prelabor rupture of membranes at term. International Journal of Gynecology & Obstetrics 2001;72:215‐21. - PubMed
-
- Hoffman RAM, Fawcus S, Anthony J. Oral misoprostol versus placebo in the management of prelabour rupture of membranes at term. Women's Health ‐ into the new millenium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town South Africa. 1999:65.
Hofmeyr 2001(T) {published and unpublished data}
-
- Hofmyer GJ, Alfirevic Z, Matonhodze B, Brocklehurst P, Campbell E, Nikodem C. Titrated oral misoprostol solution for labour induction: a multi‐centre, randomised trial. BJOG: an international journal of obstetrics and gynaecology 2001;108:952‐9. - PubMed
-
- Matonhodze B, Alfirevic Z, Hofmeyr J, Brocklehurst P. Titrated oral misoprostol for labour induction: a randomised trial [abstract]. Prenatal and Neonatal Medicine 2000;5(Suppl 2):148.
-
- Matonhodze B, Alfirevic Z, Hofmeyr J, Campbell L, Brocklehurst P. Titrated oral misoprostol for labour induction: a random allocation trial. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S19. - PubMed
-
- Matonhodze BB, Hofmeyr GJ, Levin J. Labour induction at term‐‐a randomised trial comparing Foley catheter plus titrated oral misoprostol solution, titrated oral misoprostol solution alone, and dinoprostone. South African Medical Journal 2003;93(5):375‐9. - PubMed
How 2001 (V25) {published data only}
-
- How H, Leaseburge L, Khoury J, Siddiqi T, Sibai B. Is there an ideal route of misoprostol administration for cervical ripening and labor induction [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S118. - PubMed
-
- How H, Leaseburge L, Khoury J, Siddiqi T, Spinnato J, Sibai B. A comparison of various routes and dosages of misoprostol for cervical ripening and the induction of labor. American Journal of Obstetrics and Gynecology 2001;185:911‐5. - PubMed
Javaid 2008 {published data only}
-
- Javaid MK, Hassan S, Tahira T. Management pre labour rupture of the membranes at term; induction of labor compared with expectant. Professional Medical Journal 2008;15(2):216‐9.
Jindal 2011 (V50) {published data only}
Khazardoost 2011 (V25) {published data only}
-
- Khazardoost S, Hakimi P, Noorzadeh M, Shafaat M. Misoprostol for cervical ripening: a clinical trial in 60 pregnant women. Tehran University Medical Journal 2011;68(10):595‐9.
Kipikasa 2005 {published data only}
-
- Kipikasa JH, Adair CD, Williamson J, Breen JM, Medford LK, Sanchez‐Ramos L. Use of misoprostol on an outpatient basis for postdate pregnancy. International Journal of Gynecology & Obstetrics 2005;88:108‐11. - PubMed
Kwon 2001 (V50) {published data only}
-
- Kwon J, Davies G, MacKenzie V. A comparison of oral and vaginal misoprostol for induction of labour at term: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2001;108:23‐6. - PubMed
-
- Kwon JS, Mackenzie VP, Davies GAL. A comparison of oral and vaginal misoprostol for induction of labour. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S128. - PubMed
Langenegger 2005 {published data only}
-
- Langenegger EJ, Odendaal HJ, Grove D. Oral misoprostol versus intracervical dinoprostone for induction of labour. International Journal of Gynecology & Obstetrics 2005;88:242‐8. - PubMed
le Roux 2002 (V50) {published data only}
-
- Roux P, Olarogun J, Penny J, Anthony J. Oral and vaginal misoprostol compared with dinoprostone for induction of labor: a randomized controlled trial. Obstetrics & Gynecology 2002;99(2):201‐5. - PubMed
Levy 2007 {published data only}
-
- Levy R, Vaisbuch E, Furman B, Brown D, Volach V, Hagay ZJ. Induction of labor with oral misoprostol for premature rupture of membranes at term in women with unfavorable cervix: a randomized, double‐blind, placebo‐controlled trial. Journal of Perinatal Medicine 2007;35:126‐9. - PubMed
-
- Levy R, Vaisbuch E, Furman B, Doitch H, Oron S, Hagay Z. Prospective randomized clinical trial of immediate induction of labor with oral misoprostol for prelabor rupture of the membranes in women with unfavorable cervix at term. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S44.
Lo 2003 {published data only}
-
- Lo J, Alexander J, McIntire D, Leveno K. Efficacy of oral misoprostol in nulliparous women with premature rupture of membranes. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S204.
-
- Lo J, Alexander J, McIntire D, Leveno K. Randomized trial of oral misoprostol in nulliparous women with premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S204.
-
- Lo JY, Alexander JM, McIntire DD, Leveno KJ. Ruptured membranes at term: randomized, double‐blind trial of oral misoprostol for labor induction. Obstetrics & Gynecology 2003;101(4):685‐9. - PubMed
Lyons 2001 {published data only}
-
- Lyons C, Rumney P, Huang W, Morrison E, Thomas S, Nageotte M, et al. Outpatient cervical ripening with oral misoprostol post‐term: induction rates decreased. American Journal of Obstetrics and Gynecology 2001;184(1):S116.
Majoko 2002 (V50) {published data only}
-
- Majoko F, Zwizwai M, Nystrom L, Lindmark G. Vaginal misoprostol for induction of labour: a more effective agent than prostaglandin f2 alpha gel and prostaglandin e2 pessary. Central African Journal of Medicine 2002;48(11‐12):123‐8. - PubMed
Mehrotra 2010 (V50) {published data only}
-
- Mehrotra S, Singh U, Gupta HP. A prospective double blind study using oral versus vaginal misoprostol for labour induction. Journal of Obstetrics & Gynaecology 2010;30(5):461‐4. - PubMed
Moodley 2003 {published data only}
-
- Moodley J, Venkatachalam S, Songca P. Misoprostol for cervical ripening at and near term ‐ a comparative study. South African Journal of Obstetrics and Gynaecology 2003;9(2):34‐7. - PubMed
-
- Moodley J, Venkatachalam S, Songca P. Misoprostol for cervical ripening at and near term‐‐a comparative study. South African Medical Journal 2003;93:371‐4. - PubMed
Mozurkewich 2003 {published data only}
-
- Mozurkewich E, Horrocks J, Daley S, Oeyen P, Sarvis A, Halvorson M, et al. The misoprostol study: a randomized controlled trial of misoprostol for premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S168.
-
- Mozurkewich E, Horrocks J, Daley S, Oeyen P, Halvorson M, Johnson M, et al. The MisoPROM study: a multicenter randomized comparison of oral misoprostol and oxytocin for premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 2003;189:1026‐30. - PubMed
Nagpal 2009 {published data only}
-
- Nagpal MB, Raghunandan C, Saili A. Oral misoprostol versus intracervical prostaglandin E2 gel for active management of premature rupture of membranes at term. International Journal of Gynecology & Obstetrics 2009;106(1):23‐6. - PubMed
Ngai 1996 {published data only}
-
- Ngai CSW, To WWK, Lao T, Ho PC. Cervical priming with oral misoprostol in prelabour rupture of membranes at term. 27th British Congress of Obstetrics and Gynaecology;1995 July 4‐7, Dublin, Ireland. 1995:A479.
-
- Ngai SW, To WK, Lao T, Ho PC. Cervical priming with oral misoprostol in pre‐labor rupture of membranes at term. Obstetrics & Gynecology 1996;87:923‐6. - PubMed
Ngai 2000 {published data only}
-
- Jackson N, Paterson‐Brown S. Labour characteristics and uterine activity: misoprostol compared with oxytocin in women at term with prelabour rupture of the membranes [letter]. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1181‐2. - PubMed
-
- Ngai SW, Chan YM, Lam SW, Lao T. Prospective randomised study to compare misoprostol and oxytocin for labour induction in prelabour rupture of membranes in term pregnancy. British Journal of Obstetrics and Gynaecology 1998;105 Suppl 17:82.
-
- Ngai SW, Chan YM, Lam SW, Lao TT. Labour characteristics and uterine activity: misoprostol compared with oxytocin in women at term with prelabour rupture of membranes. British Journal of Obstetrics and Gynecology 2000;107(2):222‐7. - PubMed
Nigam 2004 {published data only}
-
- Nigam A, Singh VK, Dubay P, Pandey K, Bhagioliwal A, Prakash A. Misoprostol vs. oxytocin for induction of labor at term. International Journal of Gynecology & Obstetrics 2004;86:398‐400. - PubMed
Nopdonrattkaoon 2003 (V50) {published data only}
-
- Nopdonrattakoon L. A comparison between intravaginal and oral misoprostol for labor induction: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2003;29(2):87‐91. - PubMed
Pais'wong 2008 (V25) {published data only}
-
- Paisarntantiwong R, Getgan M. A comparison between single dose of 50 microg oral misoprostol and 25 microg vaginal misoprostol for labor induction. Journal of the Medical Association of Thailand 2005;88(Suppl 2):S56‐S62. - PubMed
Patil 2005 {published data only}
-
- Patil PK, Swamy MK, Rao Radhika K. Oral misoprostol vs intra‐cervical dinoprostone for cervical ripening and labour induction. Journal of Obstetrics and Gynaecology of India 2005;55(2):128‐31.
Paungmora 2004 (V50) {published data only}
-
- Paungmora N, Herabutaya Y, O‐Prasertsawat P. A comparison of oral and vaginal misoprostol for induction of labour at term: a randomised controlled trial. Thai Journal of Obstetrics and Gynaecology 2003;15(4):272. - PubMed
-
- Paungmora N, Herabutya Y, O‐Prasertsawat P, Punyavachira P. Comparison of oral and vaginal misoprostol for induction of labor at term: a randomized controlled trial. Journal of Obstetrics & Gynaecology Research 2004;30(5):358‐62. - PubMed
Pongsatha 2001 {published data only}
-
- Pongsatha S, Tongsong T, Somsak T. A comparison between 50 mcg oral misoprostol every 4 hours and 6 hours for labor induction: a prospective randomized controlled trial. Journal of the Medical Association of Thailand 2001;84(7):989‐94. - PubMed
-
- Somsak T, Pongsatha S. A comparison between oral misoprostol 50 micrograms every 4 hours and every 6 hours for labor induction. Thai Journal of Obstetrics and Gynaecology 2000;12(4):334.
Pongsatha 2002 {published data only}
-
- Pongsatha S, Sirisukkasem S, Tongsong T. A comparison of 100ug oral misoprostol every 3 hours and 6 hours for labor induction: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2002;28(6):308‐12. - PubMed
Pongsatha 2005 (V50) {published data only}
-
- Pongsatha S, Vijittrawiwat A, Tongsong T. A comparison of labor induction by oral and vaginal misoprostol. International Journal of Gynecology & Obstetrics 2005;88:140‐1. - PubMed
Puga 2001 (V50) {published data only}
-
- Puga O, Nien J, Gomez R, Medina L, Carstens M, Gonzalez R, et al. Premature rupture of membranes after 35 weeks: a randomized clinical trial of induction of labor with oral versus vaginal administration of misoprostol. American Journal of Obstetrics and Gynecology 2001;184(1):S85.
Rahman 2013 (V25) {published data only}
-
- Rahman H. Comparative evaluation of 50ug oral misoprostol and 25ug intra‐vaginal misoprostol for induction of labour at term. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:316.
-
- Rahman H, Pradhan A, Kharka L, Renjhen P, Kar S, Dutta S. Comparative evaluation of 50 microgram oral misoprostol and 25 microgram intravaginal misoprostol for induction of labour at term: a randomized trial. Journal of Obstetrics and Gynaecology Canada : JOGC 2013;35(5):408‐16. - PubMed
Rath 2007 {published data only}
-
- Rath DM, Manas K. Induction of labor with oral misoprostol in women with prelabor rupture of membranes at term. Journal of Obstetrics and Gynecology of India 2007;57(6):505‐8.
Rizvi 2007 (V25) {published data only}
-
- Rizvi S, Umber F, Yusuf AW. Labour induction at term; oral versus intravaginal misoprostol. Annals of King Edward Medical College 2007;13(1):119‐21.
Rouzi 2014 {published data only}
-
- Rouzi AA. Randomized clinical trial between titrated oral dose of misoprostol and Propess for induction of labor. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=1261... (accessed 22 January 2013) 2011.
-
- Rouzi AA, Alsibiani S, Mansouri N, Alsinani N, Darhouse K. Randomized clinical trial between hourly titrated oral misoprostol and vaginal dinoprostone for induction of labor. American Journal of Obstetrics and Gynecology 2014;210(1):56.e1‐6. - PubMed
Schneider 2004 (V25) {published data only}
-
- Schneider M, Ramsey R, Kao L, Bennett KA. Misoprostol is effective for induction of labor in high risk pregnant women: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S73.
Sheela 2007 (V25) {published data only}
-
- Sheela CN, Mhaskar A, George S. Comparison of vaginal misoprostol and oral misoprostol with intracervical dinoprostone gel for labor induction at term. Journal of Obstetrics and Gynaecology of India 2007;57(4):327‐30.
Sheikher 2009 (V25) {published data only}
-
- Sheikher C, Suri N, Kholi U. Comparative evaluation of oral misoprostol, vaginal misoprostol and intracervical Foley's catheter for induction of labour at term. JK Science 2009;11(2):75‐7.
Shetty 2001 (V50) {published data only}
-
- Shetty A, Danielian P, Templeton A. A comparison of oral and vaginal misoprostol in the induction of labour at term: a random allocation trial. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S19. - PubMed
-
- Shetty A, Danielian P, Templeton A. A comparison of oral and vaginal misoprostol tablets in induction of labour at term. BJOG: an international journal of obstetrics and gynaecology 2001;108:238‐43. - PubMed
-
- Shetty A, Danielian P, Templeton A. A comparison of oral and vaginal tablets in the induction of labor at term. XVI FIGO World Congress of Obstetrics & Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:28‐9.
-
- Shetty A, Danielian P, Templeton A. Oral versus vaginal misoprostol in the induction of labour at term: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2000;107:813.
Shetty 2002 {published data only}
-
- Shetty A, Martin R, Danielian P, Templeton A. A comparison of two dosage regimens of oral misoprostol for labor induction at term. Acta Obstetricia et Gynecologica Scandinavica 2002;81:337‐42. - PubMed
-
- Shetty A, Martin R, Danielian P, Templeton A. A comparison of two dose regimens of oral misoprostol in the induction of labour at term: a random allocation controlled trial [abstract]. Journal of Obstetrics & Gynaecology 2001;21(1):91.
Shetty 2002a {published data only}
-
- Shetty A, Stewart K, Stewart G, Rice P, Danielian P, Templeton A. Active management of term prelabour rupture of membranes with oral misoprostol. BJOG: an international journal of obstetrics and gynaecology 2002;109:1354‐8. - PubMed
Shetty 2003 (V25) {published data only}
-
- Livingstone I, Acharya S, Shetty A, Rice P, Danielian P, Templeton A. 100ug of oral misoprostol versus 25ug of vaginal misoprostol in term labour induction ‐ a randomised comparison. Journal of Obstetrics and Gynaecology 2004;24(1):106. - PubMed
-
- Shetty A, Livingstone I, Acharya S, Rice P, Danielian P, Templeton A. Oral misoprostol (100ug) versus vaginal misoprostol (25ug) in term labor induction: a randomized comparison. Acta Obstetricia et Gynecologica Scandinavica 2003;82(12):1103‐6. - PubMed
Shetty 2004 {published data only}
-
- Shetty A, Livingstone I, Acharya S, Danielian P, Rice P, Templeton A. A randomised comparison of oral misoprostol and vaginal prostaglandin E2 tablets in labour induction at term. BJOG: an international journal of obstetrics and gynaecology 2003;110:963. - PubMed
-
- Shetty A, Livingstone I, Acharya S, Danielian P, Templeton A, Rice P. A randomised comparison of oral misoprostol and vaginal prostaglandin E2 tablets in labour induction at term. BJOG: an international journal of obstetrics and gynaecology 2004;111:436‐40. - PubMed
Sitthiwattanawong 1999 {published data only}
-
- Sitthiwattanawong W. A comparison between oral and intravaginal administration of 50 microgram misoprostol for cervical ripening and induction of labor. Thai Journal of Obstetrics and Gynaecology 2000;12(4):352.
-
- Sitthiwattanawong W, Pongsatha S. Oral misoprostol for cervical ripening and labour induction: a randomized controlled trial. Thai Journal of Obstetrics and Gynaecology 1999;11(2):87‐92.
Souza 2013(V25)(T) {published data only}
-
- Rolland Souza A. [Titrated oral suspension compared with vaginal misoprostol for labor induction: a randomized controlled trial] [Misoprostol em solução oral titulada escalonada versus via vaginal para indução do trabalho de parto: ensaio clínico randomizado]. Revista Brazileira de Ginecologia e Obstetricia 2011;33(9):270.
-
- Rolland de Souza A. Oral misoprostol titrated solution versus vaginal misoprostol for induction of labour: randomized controlled trial. http://clinicaltrials.gov/ct2/show/record/NCT00992524 (accessed 22 January 2013) 2011.
-
- Souza AS, Feitosa FE, Costa AA, Pereira AP, Carvalho AS, Paixao RM, et al. Titrated oral misoprostol solution versus vaginal misoprostol for labor induction. International Journal of Gynecology and Obstetrics 2013;123(3):207‐12. - PubMed
Sultana 2006 (V100) {published data only}
-
- Sultana N, Rouf S, Rashid M. Oral versus vaginal misoprostol for induction of labour. Journal of Bangladesh College of Physicians and Surgeons 2006;24(2):44‐9.
Tessier 1997 {published data only}
-
- * Tessier F, Danserau J. Oral misoprostol versus vaginal dinoprostone for labor induction: a double‐blind randomized controlled trial. Personal communication 1997.
-
- Tessier F, Dansereau J. A double‐blind randomized controlled trial comparing oral misoprostol to vaginal prostaglandin E2 gel for the induction of labour at or near term. American Journal of Obstetrics and Gynecology 1997;176(1):S111.
Thaisomboon 2012 (T) {published data only}
-
- Thaisomboon A, Russameecharoen K, Wanitpongpan P, Phattanachindakun B, Changnoi A. Comparison of the efficacy and safety of titrated oral misoprostol and a conventional oral regimen for cervical ripening and labor induction. International Journal of Gynecology and Obstetrics 2012;116(1):13‐6. - PubMed
Toppozada 1997 (V100 {published data only}
-
- Toppozada MK, Anwar MYM, Hassan HA, El‐Gazaerly WS. Oral or vaginal misoprostol for induction of labour. International Journal of Gynecology & Obstetrics 1997;56:135‐9. - PubMed
Uludag 2005 (V50) {published data only}
-
- Uludag S, Saricali FS, Madazli R, Cepni I. A comparison of oral and vaginal misoprostol for induction of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;122:57‐60. - PubMed
Wing 1999 (V25) {published data only}
-
- Wing D, Ham D, Paul RH. A comparison of orally administered misoprostol with vaginally administered misoprostol for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1999;180(5):1155‐60. - PubMed
-
- Wing DA, Ham D, Paul RH. A comparison of orally administered misoprostol to vaginally administered misoprostol for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S127. - PubMed
Wing 2000 (V25) {published data only}
-
- Wing DA, Park MR, Paul RH. A randomised comparison of oral and intravaginal misoprostol for labor induction. Obstetrics & Gynecology 2000;95(6 Pt 1):905‐8. - PubMed
Wing 2004 {published data only}
-
- Wing DA, Fassett MJ, Guberman C, Tran S, Parrish A, Guinn D. A comparison of orally administered misoprostol to intravenous oxytocin for labor induction in women with favorable cervix examinations. American Journal of Obstetrics and Gynecology 2004;190:1689‐96. - PubMed
References to studies excluded from this review
Abbassi 2008 {published data only}
-
- Abbassi RM, Sirichand P, Rizvi S. Safety and efficacy of oral versus vaginal misoprostol use for induction of labour at term. Journal of the College of Physicians and Surgeons Pakistan 2008;18(10):625‐9. - PubMed
Ascher‐Walsh 2000 {published data only}
-
- Ascher‐Walsh C, Burke B, Baxi L. Outpatient management of prolonged pregnancy with misoprostol: a randomised, double‐blind, placebo‐controlled study, prelim.data. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S520.
Ayaz 2008 {published data only}
-
- Ayaz A, Saeed S, Farooq MU, Ahmad F, Bahoo LA, Ahmad I. Pre‐labor rupture of membranes at term in patients with an unfavorable cervix: active versus conservative management. Taiwanese Journal of Obstetrics & Gynecology 2008;47(2):192‐6. - PubMed
Bozhinova 2007 {published data only}
-
- Bozhinova S. Is it already time to legalize the usage of cytotec (misoprostol) in the obstetrics' practice?. Akusherstvo i Ginekologiia 2007;46(9):56‐61. - PubMed
Bricker 2008 {published data only}
-
- Bricker L, Peden H, Alfirevic Z. The PROMMIS trial: a multicentre randomised trial to evaluate a low dose misoprostol regimen for induction of labour in the presence of prelabour rupture of the amniotic membranes [abstract]. Journal of Obstetrics and Gynaecology 2007;27(Suppl 1):S22‐3.
-
- Bricker L, Peden H, Tomlinson AJ, Al‐Hussaini TK, Idama T, Candelier C, et al. Titrated low‐dose vaginal and/or oral misoprostol to induce labour for prelabour membrane rupture: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2008;115(12):1503‐11. - PubMed
Delaney 2001 {published data only}
-
- Delaney T, Crane J, Hutchens D, Fanning C, Young D. Oral misoprostol labor induction in patients with a favorable cervix. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S202.
Hassan 2005 {published data only}
-
- Hassan AA. A comparison of oral misoprostol tablets and vaginal prostaglandin E2 pessary in induction of labour at term. Journal of the College of Physicians & Surgeons Pakistan (JCPSP) 2005;15(5):284‐7. - PubMed
Ho 2010 {published data only}
-
- Ho M, Cheng SY, Li TC. Titrated oral misoprostol solution compared with intravenous oxytocin for labor augmentation: a randomized controlled trial. Obstetrics & Gynecology 2010;116(3):612‐8. - PubMed
Kadanali 1996 {published data only}
-
- Kadanali S, Kucukozkan T, Zor N, Kumptepe Y. Comparison of labor induction with misoprostol versus oxytocin/prostaglandin E2 in term pregnancy. International Journal of Gynecology & Obstetrics 1996;55:99‐104. - PubMed
Neto 1988 {published data only}
-
- Neto CM, Delbin AL, Do Val Junior R. Tocographic pattern caused by misoprostol [Padrao tocografico desencadeado pelo misoprostol]. Revista Paulista de Medicina 1988;106(4):205‐8. - PubMed
Rasheed 2007 (V50) {published data only}
-
- Rasheed R, Alam AA, Younus S, Raza F. Oral versus vaginal misoprostol for labour induction. JPMA ‐ Journal of the Pakistan Medical Association 2007;57(8):404‐7. - PubMed
Robinson 2011 {published data only}
-
- Robinson D. Efficacy and safety of titrated oral misoprostol solution for labor induction at term. http://clinicaltrials.gov/ct2/show/record/NCT01070472 (accessed 22 January 2013) 2011.
Thigpen 2004 {published data only}
-
- Thigpen B, Bofill J, Bufkin L, Woodring T, Moore L, Morrison J. A randomized controlled trial comparing vaginal misoprostol to cervical foley plus oral misoprostol for cervical ripening and labor induction [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S18.
Windrim 1997 {published data only}
-
- Windrim R, Bennett K, Mundle W, Young DC. Oral administration of misoprostol for labor induction: a randomized controlled trial. Obstetrics & Gynecology 1997;89:392‐7. - PubMed
Zvandasara 2008 {published data only}
-
- Zvandasara P, Saungweme G, Mlambo J, Chidembo W, Madzivanzira N, Mwanjira C. Induction of labour with titrated oral misoprostol suspension. A comparative study with vaginal misoprostol. Central African Journal of Medicine 2008;54(9‐12):43‐9. - PubMed
References to studies awaiting assessment
Atkinson 2000 {published data only}
-
- Atkinson MW, Van Kessel, Benedetti T. The use of low dose oral misoprostol to induce labour in the third trimester. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S129.
Bonebrake 2001 {published data only}
-
- Bonebrake R, Haag T, Fleming A, Temp M, Haynatzki G. Vaginal misoprostol is more effective with fewer side effects than oral misoprostol for cervical ripening and induction of labor [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S204.
Butler 2004 {published data only}
-
- Butler B, Crane J, Delaney T. Induction of labour with misoprostol in women at term with an unfavorable cervix: a randomized comparison of oral and vaginal administration. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S190.
Getgan 2003 {published data only}
-
- Getgan M, Paisarntantiwong R, Sripramote M. A randomized comparison between 50 micrograms orally and misoprostol 25 micrograms vaginally for cervical ripening and induction of labor. Thai Journal of Obstetrics and Gynaecology 2003;15(4):276.
Goedken 2000 {published data only}
-
- Goedken J, Poehlmann S, Paul M. A blinded randomized controlled trial of misoprostol, dinoprostone, and oxytocin for labor induction. Obstetrics & Gynecology 2000;95(4 Suppl):73S.
Madhavi 2011 {published data only}
-
- Madhavi N, Jahan A. Prospective randomised comparative study of labour with misoprostol vs oxytocin in pre labour rupture of membranes. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:106.
Niroomanesh 2011 {published data only}
-
- Niroomanesh S, Dadashaliha M, Akrami M. Titrated oral misoprostol solution compared with oxytocin for induction of labor in women with unfavorable cervix. Tehran University Medical Journal 2011;69(7):413‐9.
Pearson 2002 {published data only}
-
- Pearson M, Hollier L, Shah A, Yeomans E. A randomized comparison of oral misoprostol versus intravenous oxytocin for induction of labor with term premature rupture of membranes. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S174.
Saldivar 2001 {published data only}
-
- Saldivar D, Triana H, Soria A, Guzman A, Cabero L, Farran I, et al. Oral misoprostol versus intracervical dinoprostone for induction of labour in women with an unfavourable cervix [Misoprostol oral vs dinoprostona intracervical en la induccion del trabajo en pacientes con cervix desfavorable]. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 1):293.
Tuipae 1999 {published data only}
-
- Tuipae S, Khooarmornpattana S. Effectiveness of oral misoprostol for cervical priming in term pre‐labor rupture of membranes (PROM). Thai Journal of Obstetrics and Gynaecology 1999;11(4):276.
Vijitrawiwat 2003 {published data only}
-
- Vijitrawiwat A, Pongsatha S. A comparison between oral misoprostol 100 micrograms every 3 hours and vaginal misoprostol 50 micrograms every 4 hours for labor induction. Thai Journal of Obstetrics and Gynaecology 2003;15(4):285.
Yazdani 2012 {published data only}
-
- Yazdani SH, Bouzari Z, Farahi S, Tabary AM. Oral misoprostol with oxytocin versus oxytocin alone for labor induction in pre‐labor rupture of membranes (PROM) at term pregnancy. Journal of Babol University of Medical Sciences 2012;14(3):7‐12.
Young 2001 {published data only}
-
- Young D, Delaney T, Armson T, Fanning C. Lower dose vaginal and oral misoprostol in labor induction ‐ rct. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S203.
References to ongoing studies
DebBarma 2013 {published data only}
-
- DebBarma AM. A comparative study of misoprostol oral versus vaginal route for induction of labour. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4251 (accessed 22 January 2013).
Gherman 2002 {published data only}
-
- Gherman R. A randomized double‐blind comparison of oral misoprostol dosing regimens for cervical ripening. Obstetrics & Gynecology 2002;99(4 Suppl):47S.
Pranuthi 2011 {published data only}
-
- Pranuthi R, Padmaja A, Padmaja P. Comparison of oral misoprostol with vaginal misoprostol for induction of labour. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:118.
Additional references
Abdel‐Aleem 2003
-
- Abdel‐Aleem H, Villar J, Gulmezoglu AM, Mostafa SA, Youssef AA, Shokry M, et al. The pharmacokinetics of the prostaglandin E1 analogue misoprostol in plasma and colostrum after postpartum oral administration. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;108(1):25‐8. - PubMed
Alfirevic 2009
Aslan 2004
-
- Aslan H, Unlu E, Agar M, Ceylan Y. Uterine rupture associated with misoprostol labor induction in women with previous cesarean delivery. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;113:45‐8. - PubMed
Bimbashi 2012
-
- Bimbashi A, Duley L, Ndoni E, Dokle A. Amniotomy plus intravenous oxytocin for induction of labour. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2012, issue 4. [DOI: 10.1002/14651858.CD009821; CD009821] - DOI
Boulvain 2005
Boulvain 2008
Bricker 2000
Costa 1993
-
- Costa SH, Vessey MP. Misoprostol and illegal abortion in Rio de Janeiro, Brazil. Lancet 1993;341:1258‐61. - PubMed
Curtis 1987
-
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. - PubMed
Fonseca 1991
-
- Fonseca W, Alencar AJC, Mota FSB, Coelho HLL. Misoprostol and congential malformation. Lancet 1991;338:56. - PubMed
French 2001
Garris 1989
-
- Garris RE, Kirkwood CF. Misoprostol: a prostaglandin E1 analogue. Clinical Pharmacology 1989;8:627‐44. - PubMed
Gherman 2001a
-
- Gherman RB, Heath T. Trial of labor after cesarean delivery: a pilot study of oral misoprostol for preinduction cervical ripening. Obstetrics & Gynecology 2001;97(Suppl 1):S68.
Gonzales 1999
-
- Gonzales CH, Marques Dias MJ. Congenital malformation in children exposed to misoprostol in utero. Frontiers in Fetal Health 1999;1(1):15.
Hapangama 2009
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2003
Hofmeyr 2009
-
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074.pub2] - DOI
Hofmeyr 2010
Howarth 2001
Hutton 2001
Jozwiak 2012
Kavanagh 2001
Kavanagh 2005
Kavanagh 2006a
Kavanagh 2006b
Keirse 1989
-
- Keirse MJNC, Chalmers I. Methods for inducing labour. In: Chalmers I, Enkin M, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:1057‐79.
Keirse 1993
-
- Keirse MJNC. Prostaglandins in preinduction cervical ripening: meta‐analysis of worldwide clinical experience. Journal of Reproductive Medicine 1993;38:89‐98. - PubMed
Kelly 2009
Kelly 2011
Kelly 2013
Kelly 2013a
Kundodyiwa 2009
-
- Kundodyiwa TW, Alfirevic Z, Weeks AD. Low‐dose oral misoprostol for induction of labor: a systematic review.. Obstet Gynecol 2009;113 (2, Pt 1):374‐383. - PubMed
Luckas 2000
Matonhodze 2005
-
- Matonhodze BB. Induction of labour in an under‐resourced environment. Johannesburg, South Africa: University of Witwatersrand, 2005.
Muzonzini 2004
Nishi 2013
Pastuszak 1998
-
- Pastuszak AL, Schuler L, Speck‐Martins CE, Coelho KFA, Cordello S, Vargas F, et al. Use of misoprostol during pregnancy and Mobious' syndrome in infants. New England Journal of Medicine 1998;338:1881‐5. - PubMed
Philip 2002
-
- Philip NM, Shannon C, Winikoff B. Misoprostol and teratogenicity: reviewing the evidence. Critical Issues in Reproductive Health. New York: Population Council, 2002.
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Smith 2003
Smith 2013
Thomas 2001
Weeks 2005
-
- Weeks AD, Fiala C, Safar P. Misoprostol and the debate over off‐label drug use. BJOG: an international journal of obstetrics and gynaecology 2005;112:269‐72. - PubMed
WHO 2011
-
- WHO. WHO Recommendations for Induction of Labour. Geneva: World Health Organization, 2011. - PubMed
References to other published versions of this review
Alfirevic 2001
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

