The role of functional dopamine-transporter SPECT imaging in parkinsonian syndromes, part 2
- PMID: 24924549
- PMCID: PMC7965658
- DOI: 10.3174/ajnr.A3971
The role of functional dopamine-transporter SPECT imaging in parkinsonian syndromes, part 2
Abstract
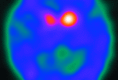
The functional imaging technique most widely used in European clinics to differentiate a true parkinsonian syndrome from vascular parkinsonism, drug-induced changes, or essential tremor is dopamine-transporter SPECT. This technique commonly reports dopamine-transporter function, with decreasing striatal uptake demonstrating increasingly severe disease. The strength of dopamine-transporter SPECT is that nigrostriatal degeneration is observed in both clinically inconclusive parkinsonism and early, even premotor, disease. In this clinical review (Part 2), we present the dopamine-transporter SPECT findings in a variety of neurodegenerative diseases, including multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration, and dementia with Lewy bodies. The findings in vascular parkinsonism, drug-induced parkinsonism, and essential tremor are also described. It is hoped that this technique will be the forerunner of a range of routinely used, process-specific ligands that can identify early degenerative disease and subsequently guide disease-modifying interventions.
© 2015 by American Journal of Neuroradiology.
Figures











References
-
- Scherfler C, Schwarz J, Antonini A, et al. . Role of DAT-SPECT in the diagnostic work-up of parkinsonism. Mov Disord 2007;22:1229–38 - PubMed
-
- Catafau AM, Tolosa E. Impact of dopamine transporter SPECT using 123I-Ioflupane on diagnosis and management of patients with clinically uncertain parkinsonian syndromes. Mov Disord 2004;19:1175–82 - PubMed
-
- Papp MI, Lantos PL. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain 1994;117:235–43 - PubMed
-
- Schrag A, Good CD, Miszkiel K, et al. . Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology 2000;54:697–702 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
