Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways
- PMID: 24924746
- PMCID: PMC4121637
- DOI: 10.1152/ajpgi.00066.2014
Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways
Abstract
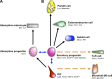
For decades, the rapid proliferation and well-defined cellular lineages of the small intestinal epithelium have driven an interest in the biology of the intestinal stem cells (ISCs) and progenitors that produce the functional cells of the epithelium. Recent and significant advances in ISC biomarker discovery have established the small intestinal epithelium as a powerful model system for studying general paradigms in somatic stem cell biology and facilitated elegant genetic and functional studies of stemness in the intestine. However, this newfound wealth of ISC biomarkers raises important questions of marker specificity. Furthermore, the ISC field must now begin to reconcile biomarker status with functional stemness, a challenge that is made more complex by emerging evidence that cellular hierarchies in the intestinal epithelium are more plastic than previously imagined, with some progenitor populations capable of dedifferentiating and functioning as ISCs following damage. In this review, we discuss the state of the ISC field in terms of biomarkers, tissue dynamics, and cellular hierarchies, and how these processes might be informed by earlier studies into signaling networks in the small intestine.
Keywords: Bmp signaling; Wnt signaling; cell fate; differentiation; intestinal stem cells.
Copyright © 2014 the American Physiological Society.
Figures



References
-
- The North Carolina Central Cancer Registry, State Center for Health Statistics. Colon and Rectum Cancer. The North Carolina Central Cancer Registry, State Center for Health Statistics. Raleigh, NC: North Carolina Public Health; http://www.schs.state.nc.us/schs/pdf/Colon_Rectum_Cancer_2011.pdf [September 6, 2012, 2012].
-
- Auclair BA, Benoit YD, Rivard N, Mishina Y, Perreault N. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology 133: 887–896, 2007 - PubMed
-
- Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138: 1681–1696, 2010 - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

