Oxidative stress-mediated aldehyde adduction of GRP78 in a mouse model of alcoholic liver disease: functional independence of ATPase activity and chaperone function
- PMID: 24924946
- PMCID: PMC4395467
- DOI: 10.1016/j.freeradbiomed.2014.06.002
Oxidative stress-mediated aldehyde adduction of GRP78 in a mouse model of alcoholic liver disease: functional independence of ATPase activity and chaperone function
Abstract
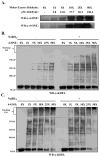
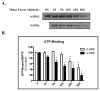
Pathogenesis in alcoholic liver disease (ALD) is complicated and multifactorial but clearly involves oxidative stress and inflammation. Currently, conflicting reports exist regarding the role of endoplasmic reticulum (ER) stress in the etiology of ALD. The glucose-regulated protein 78 (GRP78) is the ER homolog of HSP70 and plays a critical role in the cellular response to ER stress by serving as a chaperone assisting protein folding and by regulating the signaling of the unfolded protein response (UPR). Comprising three functional domains, an ATPase, a peptide-binding, and a lid domain, GRP78 folds nascent polypeptides via the substrate-binding domain. Earlier work has indicated that the ATPase function of GRP78 is intrinsically linked and essential to its chaperone activity. Previous work in our laboratory has indicated that GRP78 and the UPR are not induced in a mouse model of ALD but that GRP78 is adducted by the lipid electrophiles 4-hydroxynonenal (4-HNE) and 4-oxononenal (4-ONE) in vivo. As impairment of GRP78 has the potential to contribute to pathogenesis in ALD, we investigated the functional consequences of aldehyde adduction on GRP78 function. Identification of 4-HNE and 4-ONE target residues in purified human GRP78 revealed a marked propensity for Lys and His adduction within the ATPase domain and a relative paucity of adduct formation within the peptide-binding domain. Consistent with these findings, we observed a concomitant dose-dependent decrease in ATP-binding and ATPase activity without any discernible impairment of chaperone function. Collectively, our data indicate that ATPase activity is not essential for GRP78-mediated chaperone activity and is consistent with the hypothesis that ER stress does not play a primary initiating role in the early stages of ALD.
Keywords: 4-HNE; 4-ONE; Alcoholic liver disease; Electrophile; Free radicals; HSP; Protein oxidation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
-
- Albano E. Alcohol, oxidative stress and free radical damage. The Proceedings of the Nutrition Society. 2006;65:278–290. - PubMed
-
- Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends in molecular medicine. 2003;9:169–176. - PubMed
-
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxidants & redox signaling. 2007;9:2277–2293. - PubMed
-
- Mottaran E, Stewart SF, Rolla R, Vay D, Cipriani V, Moretti M, Vidali M, Sartori M, Rigamonti C, Day CP, Albano E. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free radical biology & medicine. 2002;32:38–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1TR001082/TR/NCATS NIH HHS/United States
- R01 AA009300/AA/NIAAA NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- R01DK074487-01/DK/NIDDK NIH HHS/United States
- UL1RR025780/RR/NCRR NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- R37AA09300/AA/NIAAA NIH HHS/United States
- P30CA046934/CA/NCI NIH HHS/United States
- F31 AA018606/AA/NIAAA NIH HHS/United States
- F31AA018606-01/AA/NIAAA NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- R01 DK074487/DK/NIDDK NIH HHS/United States
- R37 AA009300/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

