Fluorescence-guided surgery in combination with UVC irradiation cures metastatic human pancreatic cancer in orthotopic mouse models
- PMID: 24924955
- PMCID: PMC4055701
- DOI: 10.1371/journal.pone.0099977
Fluorescence-guided surgery in combination with UVC irradiation cures metastatic human pancreatic cancer in orthotopic mouse models
Abstract
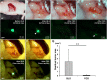
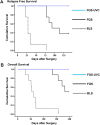
The aim of this study is to determine if ultraviolet light (UVC) irradiation in combination with fluorescence-guided surgery (FGS) can eradicate metastatic human pancreatic cancer in orthotopic nude-mouse models. Two weeks after orthotopic implantation of human MiaPaCa-2 pancreatic cancer cells, expressing green fluorescent protein (GFP), in nude mice, bright-light surgery (BLS) was performed on all tumor-bearing mice (n = 24). After BLS, mice were randomized into 3 treatment groups; BLS-only (n = 8) or FGS (n = 8) or FGS-UVC (n = 8). The residual tumors were resected using a hand-held portable imaging system under fluorescence navigation in mice treated with FGS and FGS-UVC. The surgical resection bed was irradiated with 2700 J/m2 UVC (254 nm) in the mice treated with FGS-UVC. The average residual tumor area after FGS (n = 16) was significantly smaller than after BLS only (n = 24) (0.135±0.137 mm2 and 3.338±2.929 mm2, respectively; p = 0.007). The BLS treated mice had significantly reduced survival compared to FGS- and FGS-UVC-treated mice for both relapse-free survival (RFS) (p<0.001 and p<0.001, respectively) and overall survival (OS) (p<0.001 and p<0.001, respectively). FGS-UVC-treated mice had increased RFS and OS compared to FGS-only treated mice (p = 0.008 and p = 0.025, respectively); with RFS lasting at least 150 days indicating the animals were cured. The results of the present study suggest that UVC irradiation in combination with FGS has clinical potential to increase survival.
Conflict of interest statement
Figures



Similar articles
-
Fluorescence-guided surgery of retroperitoneal-implanted human fibrosarcoma in nude mice delays or eliminates tumor recurrence and increases survival compared to bright-light surgery.PLoS One. 2015 Feb 24;10(2):e0116865. doi: 10.1371/journal.pone.0116865. eCollection 2015. PLoS One. 2015. PMID: 25710463 Free PMC article.
-
Ratiometric activatable cell-penetrating peptides label pancreatic cancer, enabling fluorescence-guided surgery, which reduces metastases and recurrence in orthotopic mouse models.Ann Surg Oncol. 2015;22(6):2082-7. doi: 10.1245/s10434-014-4144-1. Epub 2014 Oct 16. Ann Surg Oncol. 2015. PMID: 25319581 Free PMC article.
-
Fluorescence-guided surgery with a fluorophore-conjugated antibody to carcinoembryonic antigen (CEA), that highlights the tumor, improves surgical resection and increases survival in orthotopic mouse models of human pancreatic cancer.Ann Surg Oncol. 2014 Apr;21(4):1405-11. doi: 10.1245/s10434-014-3495-y. Epub 2014 Feb 6. Ann Surg Oncol. 2014. PMID: 24499827 Free PMC article.
-
Toward Curative Fluorescence-Guided Surgery of Pancreatic Cancer.Hepatogastroenterology. 2015 May;62(139):715-22. Hepatogastroenterology. 2015. PMID: 26897960 Review.
-
Advantages of patient-derived orthotopic mouse models and genetic reporters for developing fluorescence-guided surgery.J Surg Oncol. 2018 Aug;118(2):253-264. doi: 10.1002/jso.25150. Epub 2018 Aug 6. J Surg Oncol. 2018. PMID: 30080930 Free PMC article. Review.
Cited by
-
Application of GFP imaging in cancer.Lab Invest. 2015 Apr;95(4):432-52. doi: 10.1038/labinvest.2014.154. Epub 2015 Feb 16. Lab Invest. 2015. PMID: 25686095 Free PMC article. Review.
-
Eradication of melanoma in vitro and in vivo via targeting with a Killer-Red-containing telomerase-dependent adenovirus.Cell Cycle. 2017 Aug 18;16(16):1502-1508. doi: 10.1080/15384101.2016.1249548. Epub 2017 Jan 5. Cell Cycle. 2017. PMID: 28055296 Free PMC article.
-
Near infrared photoimmunotherapy in the treatment of pleural disseminated NSCLC: preclinical experience.Theranostics. 2015 Mar 19;5(7):698-709. doi: 10.7150/thno.11559. eCollection 2015. Theranostics. 2015. PMID: 25897335 Free PMC article.
-
Effective fluorescence-guided surgery of liver metastasis using a fluorescent anti-CEA antibody.J Surg Oncol. 2016 Dec;114(8):951-958. doi: 10.1002/jso.24462. Epub 2016 Oct 3. J Surg Oncol. 2016. PMID: 27696448 Free PMC article.
-
A Fluorescence-Guided Laser Ablation System for Removal of Residual Cancer in a Mouse Model of Soft Tissue Sarcoma.Theranostics. 2016 Jan 1;6(2):155-66. doi: 10.7150/thno.13536. eCollection 2016. Theranostics. 2016. PMID: 26877775 Free PMC article.
References
-
- Kato K, Yamada S, Sugimoto H, Kanazumi N, Nomoto S, et al. (2009) Prognostic factors for survival after extended pancreatectomy for pancreatic head cancer: influence of resection margin status on survival. Pancreas 38: 605–612. - PubMed
-
- Bouvet M, Hoffman RM (2011) Glowing tumors make for better detection and resection. Sci Transl Med 3: 110fs110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

