Early severe HIV disease precedes early antiretroviral therapy in infants: Are we too late?
- PMID: 24925044
- PMCID: PMC4056161
- DOI: 10.7448/IAS.17.1.18914
Early severe HIV disease precedes early antiretroviral therapy in infants: Are we too late?
Abstract
Objective: To describe the degree of HIV disease progression in infants initiating antiretroviral therapy (ART) by three months of age in a programmatic setting in South Africa.
Design: This was a programmatic cohort study.
Methods: Electronic and manual data extraction from databases and antiretroviral registers in 20 public clinics in Cape Town and electronic data extraction from a large ART service at Chris Hani Baragwanath Hospital in Soweto were performed. Records of all infants initiated on ART by three months of age between June 2007 and September 2010 were extracted. Demographics, immunological and clinical stage at ART initiation were analyzed descriptively by chi-square, two-sample t-test and Kaplan-Meier methods.
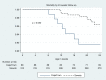
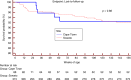
Results: A total of 403 records were identified: 88 in Cape Town and 315 in Soweto. Median age at ART initiation was 8.4 [interquartile range (IQR): 7.2-9.7] weeks. At ART initiation, 250 infants (62%) had advanced HIV disease (CD4% <25% or absolute CD4<1500 cells/mm(3) or WHO clinical Stage 3 or 4). Median age at ART initiation by site was 10.3 (IQR: 8.2-11.9) weeks in Cape Town and 8.6 (IQR: 7.7-10.0) weeks in Soweto infants (p<0.0001). In Cape Town, 73 infants (83%) had advanced HIV disease at ART initiation, compared to 177 infants (56%) in Soweto (p<0.0001). On logistic regression, each month increase in age at ART initiation lowered the odds of initiating ART in an optimal state (OR: 0.56, CI: 0.36-0.94) and increased the odds of advanced HIV disease at ART initiation (OR: 1.69, CI: 1.05-2.71).
Conclusions: ART initiation by three months of age may not adequately prevent disease progression. New emphasis on early diagnosis and rapid initiation of ART in the first weeks of life are essential to further reduce infant mortality.
Keywords: South Africa; antiretroviral therapy; early infant diagnosis; infant HIV; programmatic cohort.
Figures
References
-
- UNAIDS, World Health Organization. UNICEF. GLOBAL HIV/AIDS RESPONSE Progress Report 2011: epidemic update and health sector progress towards universal access. Geneva: WHO Press; 2011.
-
- Goga A, Dinh T, Jackson D, for the SAPMTCTE study group . Pretoria: South African Medical Research Council, National Department of Health of South Africa, and PEPFAR/US Centers for Disease Control and Prevention. 2012. Evaluation of the effectiveness of the national prevention of mother-to-child transmission (PMTCT) programme measured at six weeks postpartum in South Africa, 2010.
-
- Sherman G, Lilian R. Early infant diagnosis of HIV infection in South Africa: 2008 to 2010. Johannesburg: National Health Laboratory Service; 2011.
-
- Gortmaker SL, Hughes M, Cervia J, Brady M, Johnson GM, Seage GR, 3rd, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345(21):1522–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1U19AI53217-01/AI/NIAID NIH HHS/United States
- 5U01AI069521-02/AI/NIAID NIH HHS/United States
- 5U01AI069521-03/AI/NIAID NIH HHS/United States
- U19 AI053217/AI/NIAID NIH HHS/United States
- U01 AI069521/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- 5U01AI069521-01/AI/NIAID NIH HHS/United States
- WTX055734/WT_/Wellcome Trust/United Kingdom
- P30 AI036214-16/AI/NIAID NIH HHS/United States
- R24-TW007988-01/TW/FIC NIH HHS/United States
- AI10304442/AI/NIAID NIH HHS/United States
- R24 TW007988/TW/FIC NIH HHS/United States
- 5U01AI069521-04/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

