POMK mutations disrupt muscle development leading to a spectrum of neuromuscular presentations
- PMID: 24925318
- PMCID: PMC4189906
- DOI: 10.1093/hmg/ddu296
POMK mutations disrupt muscle development leading to a spectrum of neuromuscular presentations
Abstract
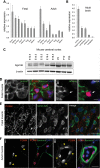
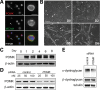
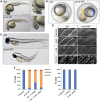
Dystroglycan is a transmembrane glycoprotein whose interactions with the extracellular matrix (ECM) are necessary for normal muscle and brain development, and disruptions of its function lead to dystroglycanopathies, a group of congenital muscular dystrophies showing extreme genetic and clinical heterogeneity. Specific glycans bound to the extracellular portion of dystroglycan, α-dystroglycan, mediate ECM interactions and most known dystroglycanopathy genes encode glycosyltransferases involved in glycan synthesis. POMK, which was found mutated in two dystroglycanopathy cases, is instead involved in a glycan phosphorylation reaction critical for ECM binding, but little is known about the clinical presentation of POMK mutations or of the function of this protein in the muscle. Here, we describe two families carrying different truncating alleles, both removing the kinase domain in POMK, with different clinical manifestations ranging from Walker-Warburg syndrome, the most severe form of dystroglycanopathy, to limb-girdle muscular dystrophy with cognitive defects. We explored POMK expression in fetal and adult human muscle and identified widespread expression primarily during fetal development in myocytes and interstitial cells suggesting a role for this protein during early muscle differentiation. Analysis of loss of function in the zebrafish embryo and larva showed that pomk function is necessary for normal muscle development, leading to locomotor dysfuction in the embryo and signs of muscular dystrophy in the larva. In summary, we defined diverse clinical presentations following POMK mutations and showed that this gene is necessary for early muscle development.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Muntoni F., Torelli S., Wells D.J., Brown S.C. Muscular dystrophies due to glycosylation defects. Curr. Opin. Neurol. 2011;24:437–442. - PubMed
-
- Cormand B., Pihko H., Bayés M., Valanne L., Santavuori P., Talim B., Gershoni-Baruch R., Ahmad A., Van Bokhoven H., Brunner H.G., et al. Clinical and genetic distinction between Walker-Warburg syndrome and muscle-eye-brain disease. Neurology. 2001;56:1059–1069. - PubMed
-
- Dobyns W.B., Pagon R.A., Armstrong D., Curry C.J., Greenberg F., Grix A., Holmes L.B., Laxova R., Michels V.V., Robinow M. Diagnostic criteria for Walker-Warburg syndrome. Am. J. Med. Genet. 1989;32:195–210. - PubMed
-
- Balci B., Uyanik G., Dincer P., Gross C., Willer T., Talim B., Haliloglu G., Kale G., Hehr U., Winkler J., et al. An autosomal recessive limb girdle muscular dystrophy (LGMD2) with mild mental retardation is allelic to Walker-Warburg syndrome (WWS) caused by a mutation in the POMT1 gene. Neuromuscular Disord. 2005;15:271–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

