ABA renewal involves enhancements in both GluA2-lacking AMPA receptor activity and GluA1 phosphorylation in the lateral amygdala
- PMID: 24925360
- PMCID: PMC4055738
- DOI: 10.1371/journal.pone.0100108
ABA renewal involves enhancements in both GluA2-lacking AMPA receptor activity and GluA1 phosphorylation in the lateral amygdala
Abstract
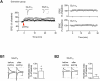
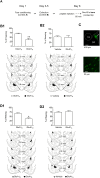
Fear renewal, the context-specific relapse of fear following fear extinction, is a leading animal model of post-traumatic stress disorders (PTSD) and fear-related disorders. Although fear extinction can diminish fear responses, this effect is restricted to the context where the extinction is carried out, and the extinguished fear strongly relapses when assessed in the original acquisition context (ABA renewal) or in a context distinct from the conditioning and extinction contexts (ABC renewal). We have previously identified Ser831 phosphorylation of GluA1 subunit in the lateral amygdala (LA) as a key molecular mechanism for ABC renewal. However, molecular mechanisms underlying ABA renewal remain to be elucidated. Here, we found that both the excitatory synaptic efficacy and GluA2-lacking AMPAR activity at thalamic input synapses onto the LA (T-LA synapses) were enhanced upon ABA renewal. GluA2-lacking AMPAR activity was also increased during low-threshold potentiation, a potential cellular substrate of renewal, at T-LA synapses. The microinjection of 1-naphtylacetyl-spermine (NASPM), a selective blocker of GluA2-lacking AMPARs, into the LA attenuated ABA renewal, suggesting a critical role of GluA2-lacking AMPARs in ABA renewal. We also found that Ser831 phosphorylation of GluA1 in the LA was increased upon ABA renewal. We developed a short peptide mimicking the Ser831-containing C-tail region of GluA1, which can be phosphorylated upon renewal (GluA1S); thus, the phosphorylated GluA1S may compete with Ser831-phosphorylated GluA1. This GluA1S peptide blocked the low-threshold potentiation when dialyzed into a recorded neuron. The microinjection of a cell-permeable form of GluA1S peptide into the LA attenuated ABA renewal. In support of the GluA1S experiments, a GluA1D peptide (in which the serine at 831 is replaced with a phosphomimetic amino acid, aspartate) attenuated ABA renewal when microinjected into the LA. These findings suggest that enhancements in both the GluA2-lacking AMPAR activity and GluA1 phosphorylation at Ser831 are required for ABA renewal.
Conflict of interest statement
Figures






Similar articles
-
GluA1 phosphorylation at serine 831 in the lateral amygdala is required for fear renewal.Nat Neurosci. 2013 Oct;16(10):1436-44. doi: 10.1038/nn.3491. Epub 2013 Aug 25. Nat Neurosci. 2013. PMID: 23974710
-
Dynamic Regulation of AMPAR Phosphorylation In Vivo Following Acute Behavioral Stress.Cell Mol Neurobiol. 2016 Nov;36(8):1331-1342. doi: 10.1007/s10571-016-0332-9. Epub 2016 Jan 27. Cell Mol Neurobiol. 2016. PMID: 26814839 Free PMC article.
-
Calcineurin regulates synaptic Ca2+-permeable AMPA receptors in hypothalamic presympathetic neurons via α2δ-1-mediated GluA1/GluA2 assembly.J Physiol. 2024 May;602(10):2179-2197. doi: 10.1113/JP286081. Epub 2024 Apr 17. J Physiol. 2024. PMID: 38630836 Free PMC article.
-
Amygdala depotentiation and fear extinction.Proc Natl Acad Sci U S A. 2007 Dec 26;104(52):20955-60. doi: 10.1073/pnas.0710548105. Epub 2007 Dec 26. Proc Natl Acad Sci U S A. 2007. PMID: 18165656 Free PMC article.
-
The Membrane Proximal Region of AMPA Receptors in Lateral Amygdala is Essential for Fear Memory Formation.Neuropsychopharmacology. 2015 Nov;40(12):2727-35. doi: 10.1038/npp.2015.121. Epub 2015 Apr 27. Neuropsychopharmacology. 2015. PMID: 25915472 Free PMC article.
Cited by
-
Posterior parietal cortex mediates fear renewal in a novel context.Mol Brain. 2020 Feb 5;13(1):16. doi: 10.1186/s13041-020-0556-y. Mol Brain. 2020. PMID: 32024548 Free PMC article.
-
Regulation of Fear Extinction in the Basolateral Amygdala by Dopamine D2 Receptors Accompanied by Altered GluR1, GluR1-Ser845 and NR2B Levels.Front Behav Neurosci. 2017 Jun 20;11:116. doi: 10.3389/fnbeh.2017.00116. eCollection 2017. Front Behav Neurosci. 2017. PMID: 28676746 Free PMC article.
-
Brain region-specific effects of cGMP-dependent kinase II knockout on AMPA receptor trafficking and animal behavior.Learn Mem. 2016 Jul 15;23(8):435-41. doi: 10.1101/lm.042960.116. Print 2016 Aug. Learn Mem. 2016. PMID: 27421896 Free PMC article.
-
Preferential generation of Ca2+-permeable AMPA receptors by AKAP79-anchored protein kinase C proceeds via GluA1 subunit phosphorylation at Ser-831.J Biol Chem. 2019 Apr 5;294(14):5521-5535. doi: 10.1074/jbc.RA118.004340. Epub 2019 Feb 8. J Biol Chem. 2019. PMID: 30737285 Free PMC article.
-
mGluR long-term depression regulates GluA2 association with COPII vesicles and exit from the endoplasmic reticulum.EMBO J. 2017 Jan 17;36(2):232-244. doi: 10.15252/embj.201694526. Epub 2016 Nov 17. EMBO J. 2017. PMID: 27856517 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

