Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease
- PMID: 24925721
- PMCID: PMC4279736
- DOI: 10.1681/ASN.2013111213
Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease
Abstract
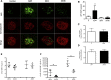
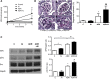
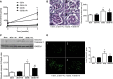
Diabetic kidney disease (DKD) is the most common cause of ESRD in the United States. Podocyte injury is an important feature of DKD that is likely to be caused by circulating factors other than glucose. Soluble urokinase plasminogen activator receptor (suPAR) is a circulating factor found to be elevated in the serum of patients with FSGS and causes podocyte αVβ3 integrin-dependent migration in vitro. Furthermore, αVβ3 integrin activation occurs in association with decreased podocyte-specific expression of acid sphingomyelinase-like phosphodiesterase 3b (SMPDL3b) in kidney biopsy specimens from patients with FSGS. However, whether suPAR-dependent αVβ3 integrin activation occurs in diseases other than FSGS and whether there is a direct link between circulating suPAR levels and SMPDL3b expression in podocytes remain to be established. Our data indicate that serum suPAR levels are also elevated in patients with DKD. However, unlike in FSGS, SMPDL3b expression was increased in glomeruli from patients with DKD and DKD sera-treated human podocytes, where it prevented αVβ3 integrin activation by its interaction with suPAR and led to increased RhoA activity, rendering podocytes more susceptible to apoptosis. In vivo, inhibition of acid sphingomyelinase reduced proteinuria in experimental DKD but not FSGS, indicating that SMPDL3b expression levels determined the podocyte injury phenotype. These observations suggest that SMPDL3b may be an important modulator of podocyte function by shifting suPAR-mediated podocyte injury from a migratory phenotype to an apoptotic phenotype and that it represents a novel therapeutic glomerular disease target.
Keywords: FSGS; diabetic nephropathy; glomerular disease; podocyte; proteinuria.
Copyright © 2015 by the American Society of Nephrology.
Figures







References
-
- Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 - PubMed
-
- Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 - PubMed
-
- White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, De Cosmo S, Viberti G: Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes 51: 3083–3089, 2002 - PubMed
-
- Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK101350/DK/NIDDK NIH HHS/United States
- 1UL-1TR00046/TR/NCATS NIH HHS/United States
- R01 DK089394/DK/NIDDK NIH HHS/United States
- R13 TR000046/TR/NCATS NIH HHS/United States
- DK090316/DK/NIDDK NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- P30 DK081943/DK/NIDDK NIH HHS/United States
- R01 DK090316/DK/NIDDK NIH HHS/United States
- R01-DK101350/DK/NIDDK NIH HHS/United States
- R01-DK073495/DK/NIDDK NIH HHS/United States
- R01 DK073495/DK/NIDDK NIH HHS/United States
- R01-DK089394/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

