Phosphodiesterase type 4 blockade prevents platelet-mediated neutrophil recruitment at the site of vascular injury
- PMID: 24925970
- PMCID: PMC4117992
- DOI: 10.1161/ATVBAHA.114.303939
Phosphodiesterase type 4 blockade prevents platelet-mediated neutrophil recruitment at the site of vascular injury
Abstract
Objective: Platelet-neutrophil interactions play a key role in cardiovascular disease and inflammatory processes. Src family kinases mediate P-selectin glycoprotein ligand-1-Mac-1 cross talk necessary for firm platelet-neutrophil adhesion. Because Src family kinase activity can be regulated by cAMP-dependent pathways, in this work, we evaluated the role of phosphodiesterases in the signaling events that are required to sustain platelet-neutrophil interactions and neutrophil recruitment at the site of vascular injury.
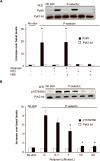
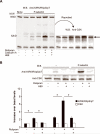
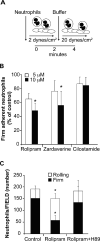
Approach and results: In neutrophils exposed to P-selectin, selective phosphodiesterase 4 (PDE4) inhibition prevented Src family kinase-mediated phosphorylation of the proline-rich tyrosine kinase 2 on Tyr579/Tyr580. The effects of PDE4 inhibition required protein kinase A, likely through protein kinase A-mediated activation of COOH-terminal Src kinase, a major negative regulator of Src family kinases. PDE4, but not other phosphodiesterase inhibitors, reduced platelet-neutrophil conjugates as well as neutrophil firm adhesion on spread platelets under flow conditions. The effect of PDE4 inhibition on neutrophil adhesion was primarily mediated by downregulation of P-selectin-induced activation of Mac-1. In a murine model of endovascular injury, selective inhibition of PDE4 significantly reduced neutrophil recruitment at the site of vascular damage.
Conclusions: This study identifies PDE4 as a central node in the signaling network that mediates platelet-neutrophil adhesion and suggests that pharmacological inhibition of PDE4 may represent a novel therapeutic avenue for the treatment of cardiovascular disease.
Keywords: neutrophils; phosphodiesterase 4 inhibitors; platelets.
© 2014 American Heart Association, Inc.
Figures



References
-
- Mickelson JK, Lakkis NM, Villarreal-Levy G, Hughes BJ, Smith CW. Leukocyte activation with platelet adhesion after coronary angioplasty: a mechanism for recurrent disease? J Am Coll Cardiol. 1996;28:345–353. - PubMed
-
- Ott I, Neumann FJ, Gawaz M, Schmitt M, Schömig A. Increased neutrophil-platelet adhesion in patients with unstable angina. Circulation. 1996;94:1239–1246. - PubMed
-
- Furman MI, Barnard MR, Krueger LA, Marsha LF, Shilale EA, Lessard DM, Marchese P, Frelinger AL, III, Goldberg LJ, Michelson AD. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38:1002–1006. - PubMed
-
- Sarma J, Laan CA, Alam S, Jha A, Fox KA, Dransfield I. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation. 2002;105:2166–2171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

