A critical role for the vascular endothelium in functional neurovascular coupling in the brain
- PMID: 24926076
- PMCID: PMC4309064
- DOI: 10.1161/JAHA.114.000787
A critical role for the vascular endothelium in functional neurovascular coupling in the brain
Abstract
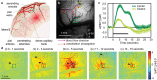
Background: The functional modulation of blood flow in the brain is critical for brain health and is the basis of contrast in functional magnetic resonance imaging. There is evident coupling between increases in neuronal activity and increases in local blood flow; however, many aspects of this neurovascular coupling remain unexplained by current models. Based on the rapid dilation of distant pial arteries during cortical functional hyperemia, we hypothesized that endothelial signaling may play a key role in the long-range propagation of vasodilation during functional hyperemia in the brain. Although well characterized in the peripheral vasculature, endothelial involvement in functional neurovascular coupling has not been demonstrated.
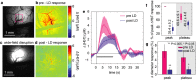
Methods and results: We combined in vivo exposed-cortex multispectral optical intrinsic signal imaging (MS-OISI) with a novel in vivo implementation of the light-dye technique to record the cortical hemodynamic response to somatosensory stimulus in rats before and after spatially selective endothelial disruption. We demonstrate that discrete interruption of endothelial signaling halts propagation of stimulus-evoked vasodilation in pial arteries, and that wide-field endothelial disruption in pial arteries significantly attenuates the hemodynamic response to stimulus, particularly the early, rapid increase and peak in hyperemia.
Conclusions: Involvement of endothelial pathways in functional neurovascular coupling provides new explanations for the spatial and temporal features of the hemodynamic response to stimulus and could explain previous results that were interpreted as evidence for astrocyte-mediated control of functional hyperemia. Our results unify many aspects of blood flow regulation in the brain and body and prompt new investigation of direct links between systemic cardiovascular disease and neural deficits.
Keywords: endothelial hyperpolarization; functional magnetic resonance imaging; neurovascular coupling; optical imaging; vascular endothelial function; vascular reactivity.
© 2014 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures


References
-
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002; 25:621-625. - PubMed
-
- D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003; 4:863-872. - PubMed
-
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006; 100:328-335. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

