Pulmonary penetration of piperacillin and tazobactam in critically ill patients
- PMID: 24926779
- PMCID: PMC4169708
- DOI: 10.1038/clpt.2014.131
Pulmonary penetration of piperacillin and tazobactam in critically ill patients
Abstract
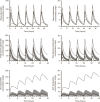
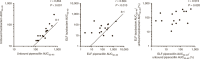
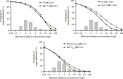
Pulmonary infections in critically ill patients are common and are associated with high morbidity and mortality. Piperacillin-tazobactam is a frequently used therapy in critically ill patients with pulmonary infection. Antibiotic concentrations in the lung reflect target-site antibiotic concentrations in patients with pneumonia. The aim of this study was to assess the plasma and intrapulmonary pharmacokinetics (PK) of piperacillin-tazobactam in critically ill patients administered standard piperacillin-tazobactam regimens. A population PK model was developed to describe plasma and intrapulmonary piperacillin and tazobactam concentrations. The probability of piperacillin exposures reaching pharmacodynamic end points and the impact of pulmonary permeability on piperacillin and tazobactam pulmonary penetration was explored. The median piperacillin and tazobactam pulmonary penetration ratios were 49.3 and 121.2%, respectively. Pulmonary piperacillin and tazobactam concentrations were unpredictable and negatively correlated with pulmonary permeability. Current piperacillin-tazobactam regimens may be insufficient to treat pneumonia caused by piperacillin-tazobactam-susceptible organisms in some critically ill patients.
Conflict of interest statement
T.W.F. is an MRC Clinical Training Fellow supported by the North West England Medical Research Council Fellowship Scheme in Clinical Pharmacology and Therapeutics, which is funded by the Medical Research Council (grant number G1000417/94909), ICON, GlaxoSmithKline, AstraZeneca, and the Medical Evaluation Unit. W.W.H. is supported by a Clinician Scientist Fellowship from the National Institute of Health Research. The other authors declared no conflict of interest.
Figures


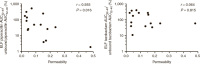
References
-
- Vincent, J.L. et al; Sepsis Occurrence in Acutely Ill Patients Investigators . Sepsis in European intensive care units: results of the SOAP study. Crit. Care Med. 34, 344–353 (2006). - PubMed
-
- Martin, G.S. , Mannino, D.M. , Eaton, S. & Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348, 1546–1554 (2003). - PubMed
-
- Vincent, J.L. et al; EPIC II Group of Investigators . International study of the prevalence and outcomes of infection in intensive care units. JAMA 302, 2323–2329 (2009). - PubMed
-
- Melsen, W.G. et al Attributable mortality of ventilator–associated pneumonia: a meta–analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 13, 665–671 (2013). - PubMed
-
- Kollef, M.H. , Shorr, A. , Tabak, Y.P. , Gupta, V. , Liu, L.Z. & Johannes, R.S. Epidemiology and outcomes of health–care–associated pneumonia: results from a large US database of culture–positive pneumonia. Chest 128, 3854–3862 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

