Capsaicin-induced activation of p53-SMAR1 auto-regulatory loop down-regulates VEGF in non-small cell lung cancer to restrain angiogenesis
- PMID: 24926985
- PMCID: PMC4057320
- DOI: 10.1371/journal.pone.0099743
Capsaicin-induced activation of p53-SMAR1 auto-regulatory loop down-regulates VEGF in non-small cell lung cancer to restrain angiogenesis
Abstract
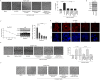
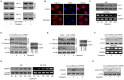
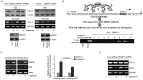
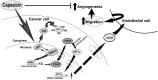
Lung cancer is the leading cause of cancer-related deaths worldwide. Despite decades of research, the treatment options for lung cancer patients remain inadequate, either to offer a cure or even a substantial survival advantage owing to its intrinsic resistance to chemotherapy. Our results propose the effectiveness of capsaicin in down-regulating VEGF expression in non-small cell lung carcinoma (NSCLC) cells in hypoxic environment. Capsaicin-treatment re-activated p53-SMAR1 positive feed-back loop in these cells to persuade p53-mediated HIF-1α degradation and SMAR1-induced repression of Cox-2 expression that restrained HIF-1α nuclear localization. Such signal-modulations consequently down regulated VEGF expression to thwart endothelial cell migration and network formation, pre-requisites of angiogenesis in tumor micro-environment. The above results advocate the candidature of capsaicin in exclusively targeting angiogenesis by down-regulating VEGF in tumor cells to achieve more efficient and cogent therapy of resistant NSCLC.
Conflict of interest statement
Figures


References
-
- Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nature reviews cancer 3: 401–410. - PubMed
-
- Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Medicine 1: 27–31. - PubMed
-
- Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nature reviews cancer 3: 721–732. - PubMed
-
- Pugh CW, Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nature medicine 9: 677–684. - PubMed
-
- Papadimitriou A, King AJ, Jones PM, Persaud SJ (2007) Anti-apoptotic effects of arachidonic acid and prostaglandin E2 in pancreatic beta-cells. Cellular physiology and biochemistry 20: 607–616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

