Redox-neutral α-sulfenylation of secondary amines: ring-fused N,S-acetals
- PMID: 24927364
- PMCID: PMC4096192
- DOI: 10.1021/ol501509b
Redox-neutral α-sulfenylation of secondary amines: ring-fused N,S-acetals
Abstract
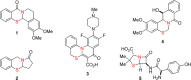
Secondary amines react with thiosalicylaldehydes in the presence of catalytic amounts of acetic acid to generate ring-fused N,S-acetals in redox-neutral fashion. A broad range of amines undergo α-sulfenylation, including challenging substrates such morpholine, thiomorpholine, and piperazines. Computational studies employing density functional theory indicate that acetic acid reduces the energy barriers of two separate steps, both of which involve proton transfer.
Figures


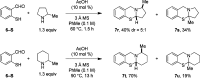


References
-
- Lombardino J. G.; McLamore W. M.; Lanbach G. D.. Derivatives of thiabenzopyrrocoline, thiabenzopyridocoline and thiazepine. US2985649A, 1961.
- Sammes P. G. Chem. Rev. 1976, 76, 113.
- Fodor L.; Szabó J.; Bernáth G.; Sohár P.; Maclean D. B.; Smith R. W.; Ninomiya I.; Naito T. J. Heterocycl. Chem. 1989, 26, 333.
- Cecchetti V.; Filipponi E.; Fravolini A.; Tabarrini O.; Xin T. Bioorg. Med. Chem. 1997, 5, 1339. - PubMed
- Deziel R.; Malenfant E. Bioorg. Med. Chem. Lett. 1998, 8, 1437. - PubMed
- Hilton S. T.; Motherwell W. B.; Potier P.; Pradet C.; Selwood D. L. Bioorg. Med. Chem. Lett. 2005, 15, 2239. - PubMed
- Song Z.-C.; Ma G.-Y.; Lv P. C.; Li H. Q.; Xiao Z. P.; Zhu H. L. Eur. J. Med. Chem. 2009, 44, 3903. - PubMed
- Gomez- Monterrey I.; Bertamino A.; Porta A.; Carotenuto A.; Musella S.; Aquino C.; Granata I.; Sala M.; Brancaccio D.; Picone D.; Ercole C.; Stiuso P.; Campiglia P.; Grieco P.; Ianelli P.; Maresca B.; Novellino E. J. Med. Chem. 2010, 53, 8319. - PubMed
- Kaur S. P.; Rao R.; Nanda S. Int. J. Pharm. Pharm. Sci. 2011, 3, 30.
-
-
Selected examples for the synthesis of ring-fused N,S-acetals:
- Marsden R.; MacLean D. B.; Fodor L. Can. J. Chem. 1984, 62, 2682.
- Okada E.; Masuda R.; Hojo M.; Tone H.; Tomifuji T. Heterocycles 1994, 37, 157.
- Hucher N.; Decroix B.; Daïch A. J. Org. Chem. 2001, 66, 4695. - PubMed
- Hucher N.; Pesquet A.; Netchitaïlo P.; Daïch A. Eur. J. Org. Chem. 2005, 2758.
- Unsworth W. P.; Kitsiou C.; Taylor R. J. K. Org. Lett. 2013, 15, 258. - PubMed
-
-
-
Other selected examples of N,S-acetal formation:
- Rothe M.; Steinberger R. Tetrahedron Lett. 1970, 11, 649.
- Rothe M.; Steinberger R. Tetrahedron Lett. 1970, 11, 2467.
- Mahata P. K.; Venkatesh C.; Syam Kumar U. K.; Ila H.; Junjappa H. J. Org. Chem. 2003, 68, 3966. - PubMed
- Ingle G. K.; Mormino M. G.; Wojtas L.; Antilla J. C. Org. Lett. 2011, 13, 4822. - PubMed
- Stojanović M.; Marković R.; Kleinpeter E.; Baranac-Stojanović M. Org. Biomol. Chem. 2012, 10, 575. - PubMed
- Li X.; Qin Z.; Yang T.; Zhang H.; Wei S.; Li C.; Chen H.; Meng M. Bioorg. Med. Chem. Lett. 2012, 22, 2712. - PubMed
-
-
-
Selected reviews on amine α-functionalization, including redox-neutral approaches:
- Murahashi S.-I. Angew. Chem., Int. Ed. Engl. 1995, 34, 2443.
- Matyus P.; Elias O.; Tapolcsanyi P.; Polonka-Balint A.; Halasz-Dajka B. Synthesis 2006, 2625.
- Campos K. R. Chem. Soc. Rev. 2007, 36, 1069. - PubMed
- Li C.-J. Acc. Chem. Res. 2009, 42, 335. - PubMed
- Jazzar R.; Hitce J.; Renaudat A.; Sofack-Kreutzer J.; Baudoin O. Chem.—Eur. J. 2010, 16, 2654. - PubMed
- Jones K. M.; Klussmann M. Synlett 2012, 23, 159.
- Pan S. C. Beilstein J. Org. Chem. 2012, 8, 1374. - PMC - PubMed
- Mitchell E. A.; Peschiulli A.; Lefevre N.; Meerpoel L.; Maes B. U. W. Chem.—Eur. J. 2012, 18, 10092. - PubMed
- Platonova A. Y.; Glukhareva T. V.; Zimovets O. A.; Morzherin Y. Y. Chem. Heterocycl. Compd. 2013, 49, 357.
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Chem. Rev. 2013, 113, 5322. - PMC - PubMed
- Peng B.; Maulide N. Chem.—Eur. J. 2013, 19, 13274. - PubMed
- Qin Y.; Lv J.; Luo S. Tetrahedron Lett. 2014, 55, 551.
- Wang L.; Xiao J. Adv. Synth. Catal. 2014, 356, 1137.
- Girard S. A.; Knauber T.; Li C.-J. Angew. Chem., Int. Ed. 2014, 53, 74. - PubMed
- Vo C.-V. T.; Bode J. W. J. Org. Chem. 2014, 79, 2809. - PubMed
- Haibach M. C.; Seidel D. Angew. Chem., Int. Ed. 2014, 53, 5010. - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

