Plant roots use a patterning mechanism to position lateral root branches toward available water
- PMID: 24927545
- PMCID: PMC4078807
- DOI: 10.1073/pnas.1400966111
Plant roots use a patterning mechanism to position lateral root branches toward available water
Abstract
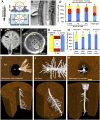
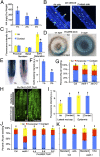
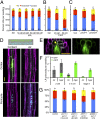
The architecture of the branched root system of plants is a major determinant of vigor. Water availability is known to impact root physiology and growth; however, the spatial scale at which this stimulus influences root architecture is poorly understood. Here we reveal that differences in the availability of water across the circumferential axis of the root create spatial cues that determine the position of lateral root branches. We show that roots of several plant species can distinguish between a wet surface and air environments and that this also impacts the patterning of root hairs, anthocyanins, and aerenchyma in a phenomenon we describe as hydropatterning. This environmental response is distinct from a touch response and requires available water to induce lateral roots along a contacted surface. X-ray microscale computed tomography and 3D reconstruction of soil-grown root systems demonstrate that such responses also occur under physiologically relevant conditions. Using early-stage lateral root markers, we show that hydropatterning acts before the initiation stage and likely determines the circumferential position at which lateral root founder cells are specified. Hydropatterning is independent of endogenous abscisic acid signaling, distinguishing it from a classic water-stress response. Higher water availability induces the biosynthesis and transport of the lateral root-inductive signal auxin through local regulation of tryptophan aminotransferase of Arabidopsis 1 and PIN-formed 3, both of which are necessary for normal hydropatterning. Our work suggests that water availability is sensed and interpreted at the suborgan level and locally patterns a wide variety of developmental processes in the root.
Keywords: adaptive root response; auxin-regulated root patterning; moisture regulation; root development; root system architecture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

