Immersive audiomotor game play enhances neural and perceptual salience of weak signals in noise
- PMID: 24927596
- PMCID: PMC4078866
- DOI: 10.1073/pnas.1322184111
Immersive audiomotor game play enhances neural and perceptual salience of weak signals in noise
Abstract
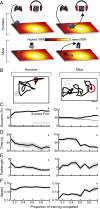
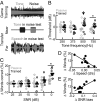
All sensory systems face the fundamental challenge of encoding weak signals in noisy backgrounds. Although discrimination abilities can improve with practice, these benefits rarely generalize to untrained stimulus dimensions. Inspired by recent findings that action video game training can impart a broader spectrum of benefits than traditional perceptual learning paradigms, we trained adult humans and mice in an immersive audio game that challenged them to forage for hidden auditory targets in a 2D soundscape. Both species learned to modulate their angular search vectors and target approach velocities based on real-time changes in the level of a weak tone embedded in broadband noise. In humans, mastery of this tone in noise task generalized to an improved ability to comprehend spoken sentences in speech babble noise. Neural plasticity in the auditory cortex of trained mice supported improved decoding of low-intensity sounds at the training frequency and an enhanced resistance to interference from background masking noise. These findings highlight the potential to improve the neural and perceptual salience of degraded sensory stimuli through immersive computerized games.
Keywords: cortical; foraging; gradient search; hearing; noise suppression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Stephens D, Krebs J. 1987. Foraging Theory (Princeton Univ Press, Princeton)
-
- Charnov EL. Optimal foraging, the marginal value theorem. Theor Popul Biol. 1976;9(2):129–136. - PubMed
-
- Greggers U, Menzel R. Memory dynamics and foraging strategies of honeybees. Behav Ecol Sociobiol. 1993;32(1):17–29.
-
- Kennedy JS. Zigzagging and casting as a programmed response to wind-borne odor—a review. Physiol Entomol. 1983;8(2):109–120.
-
- Porter J, et al. Mechanisms of scent-tracking in humans. Nat Neurosci. 2007;10(1):27–29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

