Integration of bacterial lytic polysaccharide monooxygenases into designer cellulosomes promotes enhanced cellulose degradation
- PMID: 24927597
- PMCID: PMC4078869
- DOI: 10.1073/pnas.1404148111
Integration of bacterial lytic polysaccharide monooxygenases into designer cellulosomes promotes enhanced cellulose degradation
Abstract
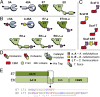
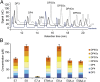
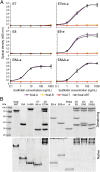
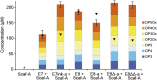
Efficient conversion of cellulose into soluble sugars is a key technological bottleneck limiting efficient production of plant-derived biofuels and chemicals. In nature, the process is achieved by the action of a wide range of cellulases and associated enzymes. In aerobic microrganisms, cellulases are secreted as free enzymes. Alternatively, in certain anaerobic microbes, cellulases are assembled into large multienzymes complexes, termed "cellulosomes," which allow for efficient hydrolysis of cellulose. Recently, it has been shown that enzymes classified as lytic polysaccharide monooxygenases (LPMOs) were able to strongly enhance the activity of cellulases. However, LPMOs are exclusively found in aerobic organisms and, thus, cannot benefit from the advantages offered by the cellulosomal system. In this study, we designed several dockerin-fused LPMOs based on enzymes from the bacterium Thermobifida fusca. The resulting chimeras exhibited activity levels on microcrystalline cellulose similar to that of the wild-type enzymes. The dockerin moieties of the chimeras were demonstrated to be functional and to specifically bind to their corresponding cohesin partner. The chimeric LPMOs were able to self-assemble in designer cellulosomes alongside an endo- and an exo-cellulase also converted to the cellulosomal mode. The resulting complexes showed a 1.7-fold increase in the release of soluble sugars from cellulose, compared with the free enzymes, and a 2.6-fold enhancement compared with free cellulases without LPMO enhancement. These results highlight the feasibility of the conversion of LPMOs to the cellulosomal mode, and that these enzymes can benefit from the proximity effects generated by the cellulosome architecture.
Keywords: biomass conversion; enzyme synergy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lynd LR, et al. How biotech can transform biofuels. Nat Biotechnol. 2008;26(2):169–172. - PubMed
-
- Ragauskas AJ, et al. The path forward for biofuels and biomaterials. Science. 2006;311(5760):484–489. - PubMed
-
- Himmel ME, Bayer EA. Lignocellulose conversion to biofuels: Current challenges, global perspectives. Curr Opin Biotechnol. 2009;20(3):316–317. - PubMed
-
- Sims REH, Mabee W, Saddler JN, Taylor M. An overview of second generation biofuel technologies. Bioresour Technol. 2010;101(6):1570–1580. - PubMed
-
- Mba Medie F, Davies GJ, Drancourt M, Henrissat B. Genome analyses highlight the different biological roles of cellulases. Nat Rev Microbiol. 2012;10(3):227–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

