Parallels between major depressive disorder and Alzheimer's disease: role of oxidative stress and genetic vulnerability
- PMID: 24927694
- PMCID: PMC4163504
- DOI: 10.1007/s10571-014-0074-5
Parallels between major depressive disorder and Alzheimer's disease: role of oxidative stress and genetic vulnerability
Abstract
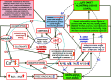
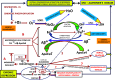
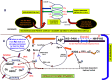
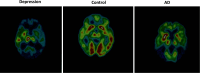
The thesis of this review is that oxidative stress is the central factor in major depressive disorder (MDD) and Alzheimer's disease (AD). The major elements involved are inflammatory cytokines, the hypothalamic-pituitary axis, the hypothalamic-pituitary gonadal, and arginine vasopressin systems, which induce glucocorticoid and "oxidopamatergic" cascades when triggered by psychosocial stress, severe life-threatening events, and mental-affective and somatic diseases. In individuals with a genomic vulnerability to depression, these cascades may result in chronic depression-anxiety-stress spectra, resulting in MDD and other known depressive syndromes. In contrast, in subjects with genomic vulnerability to AD, oxidative stress-induced brain damage triggers specific antioxidant defenses, i.e., increased levels of amyloid-β (Aβ) and aggregation of hyper-phosphorylated tau, resulting in paired helical filaments and impaired functions related to the ApoEε4 isoform, leading to complex pathological cascades culminating in AD. Surprisingly, all the AD-associated molecular pathways mentioned in this review have been shown to be similar or analogous to those found in depression, including structural damage, i.e., hippocampal and frontal cortex atrophy. Other interacting molecular signals, i.e., GSK-3β, convergent survival factors (brain-derived neurotrophic factor and heat shock proteins), and transition redox metals are also mentioned to emphasize the vast array of intermediates that could interact via comparable mechanisms in both MDD and AD.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Akiskal HS, Lewis LJ (2005) The depressive spectrum: re-conceptualizing the relationship between dysthymic, sub-threshold and major depressions. In: Licinio J, Wong M-L (eds.) Biology of depression: from novel insights to therapeutic strategies. Wiley-VCH Verlag GmbH & Co., Weinheim GmbH & Co., Weinheim, pp 47–70
-
- Akiskal HS, McKinney WTJ (1973) Depressive disorders: toward a unified hypothesis. Science 182:20–29 - PubMed
-
- Alexopoulos G, Young R, Meyers B (1993) Geriatric depression: age of onset and dementia. Biol Psychiatry 1934:141–145 - PubMed
-
- Allagy MS, Nciri R, Rouhayd MF, Mourat GC, El Feki A, Croute D, Vincent C (2009) Long term exposure to low lithium concentrations stimulates proliferation, modifies stress protein expression pattern and enhances resistance to oxidative stress in SH-SY5Y cells. Neurochem Res 34:453–462 - PubMed
-
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB (1999) The role of corticotrophin-releasing factor in depression and anxiety disorders. J Endocrinol 160:1–12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

