FW-04-806 inhibits proliferation and induces apoptosis in human breast cancer cells by binding to N-terminus of Hsp90 and disrupting Hsp90-Cdc37 complex formation
- PMID: 24927996
- PMCID: PMC4074137
- DOI: 10.1186/1476-4598-13-150
FW-04-806 inhibits proliferation and induces apoptosis in human breast cancer cells by binding to N-terminus of Hsp90 and disrupting Hsp90-Cdc37 complex formation
Abstract
Background: Heat shock protein 90 (Hsp90) is a promising therapeutic target and inhibition of Hsp90 will presumably result in suppression of multiple signaling pathways. FW-04-806, a bis-oxazolyl macrolide compound extracted from China-native Streptomyces FIM-04-806, was reported to be identical in structure to the polyketide Conglobatin.
Methods: We adopted the methods of chemproteomics, computational docking, immunoprecipitation, siRNA gene knock down, Quantitative Real-time PCR and xenograft models on the research of FW-04-806 antitumor mechanism, through the HER2-overexpressing breast cancer SKBR3 and HER2-underexpressing breast cancer MCF-7 cell line.
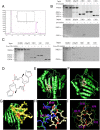
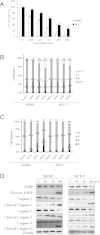
Results: We have verified the direct binding of FW-04-806 to the N-terminal domain of Hsp90 and found that FW-04-806 inhibits Hsp90/cell division cycle protein 37 (Cdc37) chaperone/co-chaperone interactions, but does not affect ATP-binding capability of Hsp90, thereby leading to the degradation of multiple Hsp90 client proteins via the proteasome pathway. In breast cancer cell lines, FW-04-806 inhibits cell proliferation, caused G2/M cell cycle arrest, induced apoptosis, and downregulated Hsp90 client proteins HER2, Akt, Raf-1 and their phosphorylated forms (p-HER2, p-Akt) in a dose and time-dependent manner. Importantly, FW-04-806 displays a better anti-tumor effect in HER2-overexpressed SKBR3 tumor xenograft model than in HER2-underexpressed MCF-7 model. The result is consistent with cell proliferation assay and in vitro apoptosis assay applied for SKBR-3 and MCF-7. Furthermore, FW-04-806 has a favorable toxicity profile.
Conclusions: As a novel Hsp90 inhibitor, FW-04-806 binds to the N-terminal of Hsp90 and inhibits Hsp90/Cdc37 interaction, resulting in the disassociation of Hsp90/Cdc37/client complexes and the degradation of Hsp90 client proteins. FW-04-806 displays promising antitumor activity against breast cancer cells both in vitro and in vivo, especially for HER2-overexpressed breast cancer cells.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

