Peripheral administration of morphine attenuates postincisional pain by regulating macrophage polarization through COX-2-dependent pathway
- PMID: 24928142
- PMCID: PMC4079829
- DOI: 10.1186/1744-8069-10-36
Peripheral administration of morphine attenuates postincisional pain by regulating macrophage polarization through COX-2-dependent pathway
Abstract
Background: Macrophage infiltration to inflammatory sites promotes wound repair and may be involved in pain hypersensitivity after surgical incision. We recently reported that the development of hyperalgesia during chronic inflammation is regulated by macrophage polarity, often referred to as proinflammatory (M1) or anti-inflammatory (M2) macrophages. Although opioids such as morphine are known to alter the inflammatory milieu of incisional wounds through interactions with immunocytes, the macrophage-mediated effects of morphine on the development of postincisional pain have not been well investigated. In this study, we examined how morphine alters pain hypersensitivity through phenotypic shifts in local macrophages during the course of incision-induced inflammation.
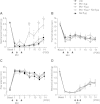
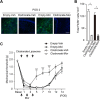
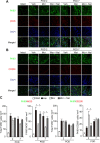
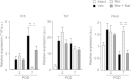
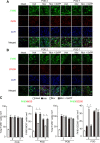
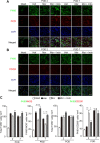
Results: Local administration of morphine in the early phase, but not in the late phase alleviated mechanical hyperalgesia, and this effect was reversed by clodronate-induced peripheral depletion of local macrophages. At the morphine-injected incisional sites, the number of pro-inflammatory F4/80+iNOS+M1 macrophages was decreased during the course of pain development whereas increased infiltration of wound healing F4/80+CD206+M2 macrophages was observed during the early phase. Morphine increased the gene expression of endogenous opioid, proenkephalin, and decreased the pronociceptive cytokine, interleukin-1β. Heme oxygenase (HO)-1 promotes the differentiation of macrophages to the M2 phenotype. An inhibitor of HO-1, tin protoporphyrin reversed morphine-induced analgesic effects and the changes in macrophage phenotype. However, local expression levels of HO-1 were not altered by morphine. Conversely, cyclooxygenase (COX)-2, primarily produced from peripheral macrophages in acute inflammation states, was up-regulated in the early phase at morphine-injected sites. In addition, the analgesic effects and a phenotype switching of infiltrated macrophages by morphine was reversed by local administration of a COX inhibitor, indomethacin.
Conclusions: Local administration of morphine alleviated the development of postincisional pain, possibly by altering macrophage polarity at the incisional sites. A morphine-induced shift in macrophage phenotype may be mediated by a COX-2-dependent mechanism. Therefore, μ-opioid receptor signaling in macrophages may be a potential therapeutic target during the early phase of postincisional pain development.
Figures



References
-
- Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth. 2010;105(Suppl 1):i69–i85. - PubMed
-
- Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, Iwakura Y, de Rivero Vaccari JP, Keane RW, Lacroix S. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: implications for neuropathic pain. J Neurosci. 2011;31:12533–12542. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

