Effects of ω3 supplementation in elderly patients with acute myocardial infarction: design of a prospective randomized placebo controlled study
- PMID: 24928284
- PMCID: PMC4074832
- DOI: 10.1186/1471-2318-14-74
Effects of ω3 supplementation in elderly patients with acute myocardial infarction: design of a prospective randomized placebo controlled study
Abstract
Background: Both epidemiological and randomized clinical studies suggest that supplementation with very-long-chain marine polyunsaturated n-3 fatty acids (n-3 PUFA) have cardioprotective effects, however these results are not without controversy. Study population, sample-size, type of supplementation and type of endpoint have all varied widely accross different studies.Therefore, the aims of the present study are to evaluate the effect of 2 years supplementation with capsules of very-long chain marine n-3 PUFA on top of standard therapy in elderly patients after acute myocardial infarction (AMI).In addition, special characteristics of this population with regard to prediction of clinical outcome will be investigated. The hypothesis is that this supplementation on top of modern therapy will reduce the occurence of major cardiovascular events (MACE). We present the design of the OMEMI (OMega-3 fatty acids in Elderly patients with Myocardial Infarction) study.
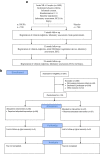
Methods/design: The OMEMI study is designed as a randomized, placebo-controlled double-blind multicenter trial.Included are patients ≥70-82 years of age who have sustained AMI. Patients of either gender are eligible. Sample size calculation based on existing literature has resulted in the need for 1400 patients followed for 2 years, based on the assumption that the n-3 PUFA supplementation will reduce MACE with 30%. The study medication is Pikasol® Axellus AS, Norway, 3 capsules (1.8 g eicosapentaenoic acid (EPA) + docohexaenoic acid (DHA)) per day, and matching placebo is corn oil. The Primary end-point is the composite of total mortality, first non-fatal recurring AMI, stroke and revascularization. Secondary end-point is the occurrence of new onset atrial fibrillation. Extensive biobanking will be performed, including adipose tissue biopsies. Compliance will be assessed by measurements of the fatty acid profile in serum, sampled at inclusion, after 12 months and at the end of study.
Discussion: The OMEMI study is scheduled to terminate when the last included patient has been followed for 2 years. To the best of our knowledge, the OMEMI study is the first to evaluate the effect of n-3 PUFAs on CVDs and mortality in a high risk elderly population having suffered an acute myocardial infarction.
Trial registration: ClinicalTrials.gov, NCT01841944.
Figures
References
-
- Mensah GA, Ryan US, Hooper WC, Engelgau MM, Callow AD, Kapuku GK, Mantovani A. Vascular endothelium summary statement II: cardiovascular disease prevention and control. Vascul Pharmacol. 2007;14:318–320. - PubMed
-
- Hagen TP, Anthun KS, Reikvam A. Acute myocardial infarctions in Norway 1. Tidsskr Nor Laegeforen. 2010;14:820–824. - PubMed
-
- Halvorsen S, Eritsland J, Abdelnoor M, Holst HC, Risoe C, Midtbo K, Bjornerheim R, Mangschau A. Gender differences in management and outcome of acute myocardial infarctions treated in 2006–2007. Cardiology. 2009;14:83–88. - PubMed
-
- Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;14:117–119. - PubMed
-
- Dyerberg J, Bang HO. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet. 1979;14:433–435. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials