Ror2 receptor mediates Wnt11 ligand signaling and affects convergence and extension movements in zebrafish
- PMID: 24928507
- PMCID: PMC4110278
- DOI: 10.1074/jbc.M114.586099
Ror2 receptor mediates Wnt11 ligand signaling and affects convergence and extension movements in zebrafish
Abstract
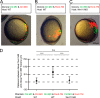
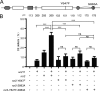
The receptor-tyrosine kinase Ror2 acts as an alternative receptor or co-receptor for Wnt5a and mediates Wnt5a-induced convergent extension movements during embryogenesis in mice and Xenopus as well as the polarity and migration of several cell types during development. However, little is known about whether Ror2 function is conserved in other vertebrates or is involved in other non-canonical Wnt ligands in vivo. In this study we demonstrated that overexpression of dominant-negative ror2 (ror2-TM) mRNA in zebrafish embryos resulted in convergence and extension defects and incompletely separated eyes, which is consistent with observations from slb/wnt11 mutants or wnt11 knockdown morphants. Moreover, the co-injection of ror2-TM mRNA and a wnt11 morpholino or the coexpression of ror2 and wnt11 in zebrafish embryos synergetically induced more severe convergence and extension defects. Transplantation studies further demonstrated that the Ror2 receptor responded to the Wnt11 ligand and regulated cell migration and cell morphology during gastrulation. DnRor2 inhibited the action of Wnt11, which was revealed by a decreased percentage of Wnt11-induced convergence and extension defects. Ror2 physically interacts with Wnt11. Theintracellular Tyr-647andSer-863 sites ofRor2are essential for mediating the action of Wnt11. Dishevelled and RhoA act downstream of Wnt11-Ror2 to regulate convergence and extension movements. Overall, our data suggest an important role of Ror2 in mediating Wnt11 signaling and in regulating convergence and extension movements in zebrafish.
Figures






Similar articles
-
Zebrafish eaf1 and eaf2/u19 mediate effective convergence and extension movements through the maintenance of wnt11 and wnt5 expression.J Biol Chem. 2009 Jun 12;284(24):16679-16692. doi: 10.1074/jbc.M109.009654. Epub 2009 Apr 20. J Biol Chem. 2009. PMID: 19380582 Free PMC article.
-
Secreted Frizzled-related Protein 2 (sFRP2) Redirects Non-canonical Wnt Signaling from Fz7 to Ror2 during Vertebrate Gastrulation.J Biol Chem. 2016 Jun 24;291(26):13730-42. doi: 10.1074/jbc.M116.733766. Epub 2016 Apr 29. J Biol Chem. 2016. PMID: 27129770 Free PMC article.
-
The PDZ domain protein Mcc is a novel effector of non-canonical Wnt signaling during convergence and extension in zebrafish.Development. 2014 Sep;141(18):3505-16. doi: 10.1242/dev.114033. Development. 2014. PMID: 25183869
-
Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases.Dev Dyn. 2010 Jan;239(1):1-15. doi: 10.1002/dvdy.21991. Dev Dyn. 2010. PMID: 19530173 Review.
-
Insight into the role of Wnt5a-induced signaling in normal and cancer cells.Int Rev Cell Mol Biol. 2015;314:117-48. doi: 10.1016/bs.ircmb.2014.10.003. Epub 2014 Nov 18. Int Rev Cell Mol Biol. 2015. PMID: 25619716 Review.
Cited by
-
Ror2 signaling regulated by differential Wnt proteins determines pathological fate of muscle mesenchymal progenitors.Cell Death Dis. 2024 Oct 29;15(10):784. doi: 10.1038/s41419-024-07173-9. Cell Death Dis. 2024. PMID: 39468010 Free PMC article.
-
Wnt/PCP controls spreading of Wnt/β-catenin signals by cytonemes in vertebrates.Elife. 2018 Jul 31;7:e36953. doi: 10.7554/eLife.36953. Elife. 2018. PMID: 30060804 Free PMC article.
-
Ror2 Signaling and Its Relevance in Breast Cancer Progression.Front Oncol. 2017 Jun 26;7:135. doi: 10.3389/fonc.2017.00135. eCollection 2017. Front Oncol. 2017. PMID: 28695110 Free PMC article.
-
Drosophila Ror is a nervous system-specific co-receptor for Wnt ligands.Biol Open. 2018 Nov 2;7(11):bio033001. doi: 10.1242/bio.033001. Biol Open. 2018. PMID: 30341100 Free PMC article.
-
The E3 ubiquitin ligase mindbomb1 controls planar cell polarity-dependent convergent extension movements during zebrafish gastrulation.Elife. 2022 Feb 10;11:e71928. doi: 10.7554/eLife.71928. Elife. 2022. PMID: 35142609 Free PMC article.
References
-
- Solnica-Krezel L. (2005) Conserved patterns of cell movements during vertebrate gastrulation. Curr. Biol. 15, R213–R228 - PubMed
-
- Heisenberg C. P., Tada M. (2002) Zebrafish gastrulation movements: bridging cell and developmental biology. Semin. Cell Dev. Biol. 13, 471–479 - PubMed
-
- Kuhl M. (2002) Non-canonical Wnt signaling in Xenopus: regulation of axis formation and gastrulation. Semin. Cell Dev. Biol. 13, 243–249 - PubMed
-
- Myers D. C., Sepich D. S., Solnica-Krezel L. (2002) Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet. 18, 447–455 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

