Biochemical and spatial coincidence in the provisional Ser/Thr protein kinase interaction network of Mycobacterium tuberculosis
- PMID: 24928517
- PMCID: PMC4110253
- DOI: 10.1074/jbc.M114.559054
Biochemical and spatial coincidence in the provisional Ser/Thr protein kinase interaction network of Mycobacterium tuberculosis
Abstract
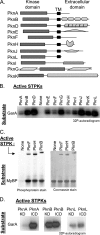
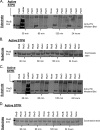
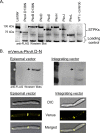
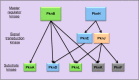
Many Gram-positive bacteria coordinate cellular processes by signaling through Ser/Thr protein kinases (STPKs), but the architecture of these phosphosignaling cascades is unknown. To investigate the network structure of a prokaryotic STPK system, we comprehensively explored the pattern of signal transduction in the Mycobacterium tuberculosis Ser/Thr kinome. Autophosphorylation is the dominant mode of STPK activation, but the 11 M. tuberculosis STPKs also show a specific pattern of efficient cross-phosphorylation in vitro. The biochemical specificity intrinsic to each kinase domain was used to map the provisional signaling network, revealing a three-layer architecture that includes master regulators, signal transducers, and terminal substrates. Fluorescence microscopy revealed that the STPKs are specifically localized in the cell. Master STPKs are concentrated at the same subcellular sites as their substrates, providing additional support for the biochemically defined network. Together, these studies imply a branched functional architecture of the M. tuberculosis Ser/Thr kinome that could enable horizontal signal spreading. This systems-level approach provides a biochemical and spatial framework for understanding Ser/Thr phospho-signaling in M. tuberculosis, which differs fundamentally from previously defined linear histidine kinase cascades.
Figures




References
-
- Wehenkel A., Bellinzoni M., Graña M., Duran R., Villarino A., Fernandez P., Andre-Leroux G., England P., Takiff H., Cerveñansky C., Cole S. T., Alzari P. M. (2008) Mycobacterial Ser/Thr protein kinases and phosphatases: physiological roles and therapeutic potential. Biochim. Biophys. Acta 1784, 193–202 - PubMed
-
- Chang L., Karin M. (2001) Mammalian MAP kinase signalling cascades. Nature 410, 37–40 - PubMed
-
- Cole S. T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S. V., Eiglmeier K., Gas S., Barry C. E., 3rd, Tekaia F., Badcock K., Basham D., Brown D., Chillingworth T., Connor R., Davies R., Devlin K., Feltwell T., Gentles S., Hamlin N., Holroyd S., Hornsby T., Jagels K., Krogh A., McLean J., Moule S., Murphy L., Oliver K., Osborne J., Quail M. A., Rajandream M. A., Rogers J., Rutter S., Seeger K., Skelton J., Squares R., Squares S., Sulston J. E., Taylor K., Whitehead S., Barrell B. G. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

