Chem-seq permits identification of genomic targets of drugs against androgen receptor regulation selected by functional phenotypic screens
- PMID: 24928520
- PMCID: PMC4078819
- DOI: 10.1073/pnas.1404303111
Chem-seq permits identification of genomic targets of drugs against androgen receptor regulation selected by functional phenotypic screens
Abstract
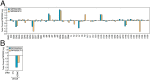
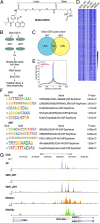
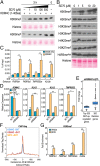
Understanding the mechanisms by which compounds discovered using cell-based phenotypic screening strategies might exert their effects would be highly augmented by new approaches exploring their potential interactions with the genome. For example, altered androgen receptor (AR) transcriptional programs, including castration resistance and subsequent chromosomal translocations, play key roles in prostate cancer pathological progression, making the quest for identification of new therapeutic agents and an understanding of their actions a continued priority. Here we report an approach that has permitted us to uncover the sites and mechanisms of action of a drug, referred to as "SD70," initially identified by phenotypic screening for inhibitors of ligand and genotoxic stress-induced translocations in prostate cancer cells. Based on synthesis of a derivatized form of SD70 that permits its application for a ChIP-sequencing-like approach, referred to as "Chem-seq," we were next able to efficiently map the genome-wide binding locations of this small molecule, revealing that it largely colocalized with AR on regulatory enhancers. Based on these observations, we performed the appropriate global analyses to ascertain that SD70 inhibits the androgen-dependent AR program, and prostate cancer cell growth, acting, at least in part, by functionally inhibiting the Jumonji domain-containing demethylase, KDM4C. Global location of candidate drugs represents a powerful strategy for new drug development by mapping genome-wide location of small molecules, a powerful adjunct to contemporary drug development strategies.
Keywords: histone demethylase; transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Drews J. Stategic trends in the drug industry. Drug Discov Today. 2003;8(9):411–420. - PubMed
-
- Bell O, Tiwari VK, Thomä NH, Schübeler D. Determinants and dynamics of genome accessibility. Nat Rev Genet. 2011;12(8):554–564. - PubMed
-
- Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet. 2011;12(1):7–18. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 NS034934/NS/NINDS NIH HHS/United States
- R01 CA173903/CA/NCI NIH HHS/United States
- PC111467/PC/NCI NIH HHS/United States
- DK18477/DK/NIDDK NIH HHS/United States
- R01 DK018477/DK/NIDDK NIH HHS/United States
- NS034934/NS/NINDS NIH HHS/United States
- R01 DK039949/DK/NIDDK NIH HHS/United States
- R00 DK094981/DK/NIDDK NIH HHS/United States
- R00 CA166527/CA/NCI NIH HHS/United States
- CA173903/CA/NCI NIH HHS/United States
- K99 DK094981/DK/NIDDK NIH HHS/United States
- 1K99DK094981-01/DK/NIDDK NIH HHS/United States
- 4R00CA166527-02/CA/NCI NIH HHS/United States
- R37 DK039949/DK/NIDDK NIH HHS/United States
- DK039949/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

