TLR signaling controls lethal encephalitis in WNV-infected brain
- PMID: 24928618
- PMCID: PMC4099315
- DOI: 10.1016/j.brainres.2014.05.049
TLR signaling controls lethal encephalitis in WNV-infected brain
Abstract
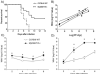
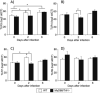
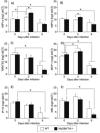
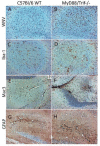
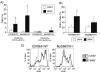
Toll-like receptors (TLRs) are known to be activated in Central Nervous System (CNS) viral infections and are recognized to be a critical component in innate immunity. Several reports state a role for particular TLRs in various CNS viral infections. However, excessive TLR activation was previously reported by us in correlation with a pathogenic, rather than a protective, outcome, in a model of SIV encephalitis. Here we aimed at understanding the impact of TLR-mediated pathways by evaluating the early course of pathogenesis in the total absence of TLR signaling during CNS viral infections. We utilized a mouse model of sublethal West Nile virus (WNV) infection. WNV is an emerging neurotropic flavivirus, and a significant global cause of viral encephalitis. The virus was peripherally injected into animals that simultaneously lacked two key adapter molecules of TLR signaling, MyD88 and TRIF. On day 2 pi (post infection), MyD88/Trif-/- mice showed an increased susceptibility to WNV infection, and revealed an impairment in innate immune cytokines, when compared to wild type mice (WT). By day 6 pi, there was an increase in viral burden and robust expression of inflammatory cytokines as well as higher cell infiltration into the CNS in MyD88/Trif-/-, when compared to infected WT. A drastic increase in microglia activation, astrogliosis, and inflammatory trafficking were also observed on day 6 pi in MyD88/Trif-/-. Our observations show a protective role for TLR signaling pathways in preventing lethal encephalitis at early stages of WNV infection.
Keywords: MyD88; Toll-like receptors (TLRs); Trif; West Nile Virus.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
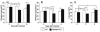
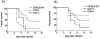
Similar articles
-
A West Nile virus NS4B-P38G mutant strain induces adaptive immunity via TLR7-MyD88-dependent and independent signaling pathways.Vaccine. 2013 Aug 28;31(38):4143-51. doi: 10.1016/j.vaccine.2013.06.093. Epub 2013 Jul 8. Vaccine. 2013. PMID: 23845800 Free PMC article.
-
The innate immune adaptor molecule MyD88 restricts West Nile virus replication and spread in neurons of the central nervous system.J Virol. 2010 Dec;84(23):12125-38. doi: 10.1128/JVI.01026-10. Epub 2010 Sep 29. J Virol. 2010. PMID: 20881045 Free PMC article.
-
Toll-Like Receptor 3 Signaling via TRIF Contributes to a Protective Innate Immune Response to Severe Acute Respiratory Syndrome Coronavirus Infection.mBio. 2015 May 26;6(3):e00638-15. doi: 10.1128/mBio.00638-15. mBio. 2015. PMID: 26015500 Free PMC article.
-
Impact of Toll-Like Receptors (TLRs) and TLR Signaling Proteins in Trigeminal Ganglia Impairing Herpes Simplex Virus 1 (HSV-1) Progression to Encephalitis: Insights from Mouse Models.Front Biosci (Landmark Ed). 2024 Mar 14;29(3):102. doi: 10.31083/j.fbl2903102. Front Biosci (Landmark Ed). 2024. PMID: 38538263 Review.
-
Microbial recognition by Toll-like receptors.J Dermatol Sci. 2004 Apr;34(2):73-82. doi: 10.1016/j.jdermsci.2003.10.002. J Dermatol Sci. 2004. PMID: 15033189 Review.
Cited by
-
Therapeutic Targeting of TLR4 for Inflammation, Infection, and Cancer: A Perspective for Disaccharide Lipid A Mimetics.Pharmaceuticals (Basel). 2022 Dec 23;16(1):23. doi: 10.3390/ph16010023. Pharmaceuticals (Basel). 2022. PMID: 36678520 Free PMC article. Review.
-
The Role of Microglia during West Nile Virus Infection of the Central Nervous System.Vaccines (Basel). 2020 Aug 28;8(3):485. doi: 10.3390/vaccines8030485. Vaccines (Basel). 2020. PMID: 32872152 Free PMC article. Review.
-
Inflammatory Response Associated with West Nile Neuroinvasive Disease: A Systematic Review.Viruses. 2024 Feb 29;16(3):383. doi: 10.3390/v16030383. Viruses. 2024. PMID: 38543749 Free PMC article.
-
Polio virotherapy targets the malignant glioma myeloid infiltrate with diffuse microglia activation engulfing the CNS.Neuro Oncol. 2023 Sep 5;25(9):1631-1643. doi: 10.1093/neuonc/noad052. Neuro Oncol. 2023. PMID: 36864784 Free PMC article.
-
Toll-like Receptors in Viral Encephalitis.Viruses. 2021 Oct 14;13(10):2065. doi: 10.3390/v13102065. Viruses. 2021. PMID: 34696494 Free PMC article. Review.
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Beasley DW, Li L, Suderman MT, Barrett AD. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology. 2002;296:17–23. - PubMed
-
- Beverley PC, Merkenschlager M, Terry L. Phenotypic diversity of the CD45 antigen and its relationship to function. Immunol Suppl. 1988;1:3–5. - PubMed
-
- Beverley PC. CD45 isoform expression: implications for recirculation of naive and memory cells. Immunol Res. 1991;10:196–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

