PARP-2 and PARP-3 are selectively activated by 5' phosphorylated DNA breaks through an allosteric regulatory mechanism shared with PARP-1
- PMID: 24928857
- PMCID: PMC4081085
- DOI: 10.1093/nar/gku474
PARP-2 and PARP-3 are selectively activated by 5' phosphorylated DNA breaks through an allosteric regulatory mechanism shared with PARP-1
Abstract
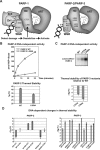
PARP-1, PARP-2 and PARP-3 are DNA-dependent PARPs that localize to DNA damage, synthesize poly(ADP-ribose) (PAR) covalently attached to target proteins including themselves, and thereby recruit repair factors to DNA breaks to increase repair efficiency. PARP-1, PARP-2 and PARP-3 have in common two C-terminal domains-Trp-Gly-Arg (WGR) and catalytic (CAT). In contrast, the N-terminal region (NTR) of PARP-1 is over 500 residues and includes four regulatory domains, whereas PARP-2 and PARP-3 have smaller NTRs (70 and 40 residues, respectively) of unknown structural composition and function. Here, we show that PARP-2 and PARP-3 are preferentially activated by DNA breaks harboring a 5' phosphate (5'P), suggesting selective activation in response to specific DNA repair intermediates, in particular structures that are competent for DNA ligation. In contrast to PARP-1, the NTRs of PARP-2 and PARP-3 are not strictly required for DNA binding or for DNA-dependent activation. Rather, the WGR domain is the central regulatory domain of PARP-2 and PARP-3. Finally, PARP-1, PARP-2 and PARP-3 share an allosteric regulatory mechanism of DNA-dependent catalytic activation through a local destabilization of the CAT. Collectively, our study provides new insights into the specialization of the DNA-dependent PARPs and their specific roles in DNA repair pathways.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Ame J.C., Spenlehauer C., de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. - PubMed
-
- Hottiger M.O., Hassa P.O., Luscher B., Schuler H., Koch-Nolte F. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends Biochem. Sci. 2010;35:208–219. - PubMed
-
- Kleine H., Poreba E., Lesniewicz K., Hassa P.O., Hottiger M.O., Litchfield D.W., Shilton B.H., Luscher B. Substrate-assisted catalysis by PARP10 limits its activity to mono-ADP-ribosylation. Mol. Cell. 2008;32:57–69. - PubMed
-
- Ame J.C., Rolli V., Schreiber V., Niedergang C., Apiou F., Decker P., Muller S., Hoger T., Menissier-de Murcia J., de Murcia G. PARP-2, A novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J. Biol. Chem. 1999;274:17860–17868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

