von Willebrand factor, Jedi knight of the bloodstream
- PMID: 24928861
- PMCID: PMC4148764
- DOI: 10.1182/blood-2014-05-378638
von Willebrand factor, Jedi knight of the bloodstream
Abstract
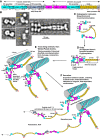
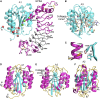
When blood vessels are cut, the forces in the bloodstream increase and change character. The dark side of these forces causes hemorrhage and death. However, von Willebrand factor (VWF), with help from our circulatory system and platelets, harnesses the same forces to form a hemostatic plug. Force and VWF function are so closely intertwined that, like members of the Jedi Order in the movie Star Wars who learn to use "the Force" to do good, VWF may be considered the Jedi knight of the bloodstream. The long length of VWF enables responsiveness to flow. The shape of VWF is predicted to alter from irregularly coiled to extended thread-like in the transition from shear to elongational flow at sites of hemostasis and thrombosis. Elongational force propagated through the length of VWF in its thread-like shape exposes its monomers for multimeric binding to platelets and subendothelium and likely also increases affinity of the A1 domain for platelets. Specialized domains concatenate and compact VWF during biosynthesis. A2 domain unfolding by hydrodynamic force enables postsecretion regulation of VWF length. Mutations in VWF in von Willebrand disease contribute to and are illuminated by VWF biology. I attempt to integrate classic studies on the physiology of hemostatic plug formation into modern molecular understanding, and point out what remains to be learned.
© 2014 by The American Society of Hematology.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

