Epithelial adhesion mediated by pilin SpaC is required for Lactobacillus rhamnosus GG-induced cellular responses
- PMID: 24928883
- PMCID: PMC4135752
- DOI: 10.1128/AEM.01039-14
Epithelial adhesion mediated by pilin SpaC is required for Lactobacillus rhamnosus GG-induced cellular responses
Abstract
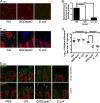
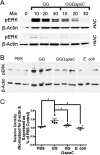
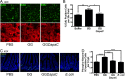
Lactobacillus rhamnosus GG is a widely used probiotic, and the strain's salutary effects on the intestine have been extensively documented. We previously reported that strain GG can modulate inflammatory signaling, as well as epithelial migration and proliferation, by activating NADPH oxidase 1-catalyzed generation of reactive oxygen species (ROS). However, how strain GG induces these responses is unknown. Here, we report that strain GG's probiotic benefits are dependent on the bacterial-epithelial interaction mediated by the SpaC pilin subunit. By comparing strain GG to an isogenic mutant that lacks SpaC (strain GGΩspaC), we establish that SpaC is necessary for strain GG to adhere to gut mucosa, that SpaC contributes to strain GG-induced epithelial generation of ROS, and that SpaC plays a role in strain GG's capacity to stimulate extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) signaling in enterocytes. In addition, we show that SpaC is required for strain GG-mediated stimulation of cell proliferation and protection against radiologically inflicted intestinal injury. The identification of a critical surface protein required for strain GG to mediate its probiotic influence advances our understanding of the molecular basis for the symbiotic relationship between some commensal bacteria of the gut lumen and enterocytes. Further insights into this relationship are critical for the development of novel approaches to treat intestinal diseases.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

