Faster cross-bridge detachment and increased tension cost in human hypertrophic cardiomyopathy with the R403Q MYH7 mutation
- PMID: 24928957
- PMCID: PMC4146374
- DOI: 10.1113/jphysiol.2014.274571
Faster cross-bridge detachment and increased tension cost in human hypertrophic cardiomyopathy with the R403Q MYH7 mutation
Abstract
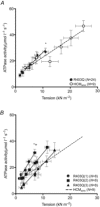
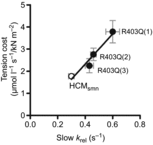
The first mutation associated with hypertrophic cardiomyopathy (HCM) is the R403Q mutation in the gene encoding β-myosin heavy chain (β-MyHC). R403Q locates in the globular head of myosin (S1), responsible for interaction with actin, and thus motor function of myosin. Increased cross-bridge relaxation kinetics caused by the R403Q mutation might underlie increased energetic cost of tension generation; however, direct evidence is absent. Here we studied to what extent cross-bridge kinetics and energetics are related in single cardiac myofibrils and multicellular cardiac muscle strips of three HCM patients with the R403Q mutation and nine sarcomere mutation-negative HCM patients (HCMsmn). Expression of R403Q was on average 41 ± 4% of total MYH7 mRNA. Cross-bridge slow relaxation kinetics in single R403Q myofibrils was significantly higher (P < 0.0001) than in HCMsmn myofibrils (0.47 ± 0.02 and 0.30 ± 0.02 s(-1), respectively). Moreover, compared to HCMsmn, tension cost was significantly higher in the muscle strips of the three R403Q patients (2.93 ± 0.25 and 1.78 ± 0.10 μmol l(-1) s(-1) kN(-1) m(-2), respectively) which showed a positive linear correlation with relaxation kinetics in the corresponding myofibril preparations. This correlation suggests that faster cross-bridge relaxation kinetics results in an increase in energetic cost of tension generation in human HCM with the R403Q mutation compared to HCMsmn. Therefore, increased tension cost might contribute to HCM disease in patients carrying the R403Q mutation.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures



References
-
- Becker E, Navarro-Lopez F, Francino A, Brenner B, Kraft T. Quantification of mutant versus wild-type myosin in human muscle biopsies using nano-LC/ESI-MS. Anal Chem. 2007;79:9531–9538. - PubMed
-
- Becker KD, Gottshall KR, Hickey R, Perriard JC, Chien KR. Point mutations in human β cardiac myosin heavy chain have differential effects on sarcomeric structure and assembly: an ATP binding site change disrupts both thick and thin filaments, whereas hypertrophic cardiomyopathy mutations display normal assembly. J Cell Biol. 1997;137:131–140. - PMC - PubMed
-
- Belus A, Piroddi N, Ferrantini C, Tesi C, Cazorla O, Toniolo L, Drost M, Mearini G, Carrier L, Rossi A, Mugelli A, Cerbai E, van der Velden J, Poggesi C. Effects of chronic atrial fibrillation on active and passive force generation in human atrial myofibrils. Circ Res. 2010;107:144–152. - PubMed
-
- Blanchard E, Seidman C, Seidman JG, LeWinter M, Maughan D. Altered crossbridge kinetics in the MHC403/+ mouse model of familial hypertrophic cardiomyopathy. Circ Res. 1999;84:475–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

