Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension
- PMID: 24928992
- PMCID: PMC4100597
- DOI: 10.4049/jimmunol.1303048
Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension
Abstract
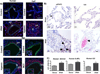
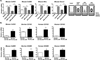
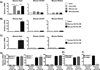
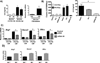
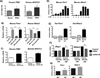
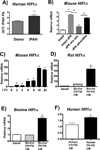
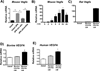
Macrophage accumulation is not only a characteristic hallmark but is also a critical component of pulmonary artery remodeling associated with pulmonary hypertension (PH). However, the cellular and molecular mechanisms that drive vascular macrophage activation and their functional phenotype remain poorly defined. Using multiple levels of in vivo (bovine and rat models of hypoxia-induced PH, together with human tissue samples) and in vitro (primary mouse, rat, and bovine macrophages, human monocytes, and primary human and bovine fibroblasts) approaches, we observed that adventitial fibroblasts derived from hypertensive pulmonary arteries (bovine and human) regulate macrophage activation. These fibroblasts activate macrophages through paracrine IL-6 and STAT3, HIF1, and C/EBPβ signaling to drive expression of genes previously implicated in chronic inflammation, tissue remodeling, and PH. This distinct fibroblast-activated macrophage phenotype was independent of IL-4/IL-13-STAT6 and TLR-MyD88 signaling. We found that genetic STAT3 haplodeficiency in macrophages attenuated macrophage activation, complete STAT3 deficiency increased macrophage activation through compensatory upregulation of STAT1 signaling, and deficiency in C/EBPβ or HIF1 attenuated fibroblast-driven macrophage activation. These findings challenge the current paradigm of IL-4/IL-13-STAT6-mediated alternative macrophage activation as the sole driver of vascular remodeling in PH, and uncover a cross-talk between adventitial fibroblasts and macrophages in which paracrine IL-6-activated STAT3, HIF1α, and C/EBPβ signaling are critical for macrophage activation and polarization. Thus, targeting IL-6 signaling in macrophages by completely inhibiting C/EBPβ or HIF1α or by partially inhibiting STAT3 may hold therapeutic value for treatment of PH and other inflammatory conditions characterized by increased IL-6 and absent IL-4/IL-13 signaling.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures

References
-
- Morrell NW, Archer SL, Defelice A, Evans S, Fiszman M, Martin T, Saulnier M, Rabinovitch M, Schermuly R, Stewart D, Truebel H, Walker G, Stenmark KR. Anticipated classes of new medications and molecular targets for pulmonary arterial hypertension. Pulmonary circulation. 2013;3:226–244. - PMC - PubMed
-
- Price LC, Wort SJ, Perros F, Dorfmuller P, Huertas A, Montani D, Cohen-Kaminsky S, Humbert M. Inflammation in pulmonary arterial hypertension. Chest. 2012;141:210–221. - PubMed
-
- Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2012;186:897–908. - PubMed
-
- Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. American journal of physiology. Lung cellular and molecular physiology. 2009;297:L1013–L1032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

