Assessing the discordance rate between local and central HER2 testing in women with locally determined HER2-negative breast cancer
- PMID: 24930388
- PMCID: PMC4232097
- DOI: 10.1002/cncr.28710
Assessing the discordance rate between local and central HER2 testing in women with locally determined HER2-negative breast cancer
Abstract
Background: The importance of human epidermal growth factor receptor 2 (HER2) as a prognostic and predictive marker in invasive breast cancer is well established. Accurate assessment of HER2 status is essential to determine optimal treatment options.
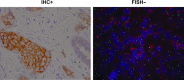
Methods: Breast cancer tumor tissue samples from the VIRGO observational cohort tissue substudy that were locally HER2-negative were retested centrally with both US Food and Drug Administration (FDA)-approved immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) assays, using FDA-approved assay cutoffs; results were compared.
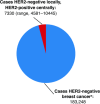
Results: Of the 552 unique patient samples centrally retested with local HER2-negative results recorded, tumor samples from 22 (4.0%) patients were determined to be HER2-positive (95% confidence interval [CI] = 2.5%-5.7%). Of these, 18 had been tested locally by only one testing methodology; 15 of 18 were HER2-positive after the central retesting, based on the testing methodology not performed locally. Compared with the 530 patients with centrally confirmed HER2-negative tumors, the 22 patients with centrally determined HER2-positive tumors were younger (median age 56.5 versus 60.0 years) and more likely to have ER/PR-negative tumors (27.3% versus 22.3%). These patients also had shorter median progression-free survival (6.4 months [95% CI = 3.8-15.9 months] versus 9.1 months [95% CI = 8.3-10.3 months]) and overall survival (25.9 months [95% CI = 13.8-not estimable] versus 27.9 months [95% CI = 25.0-32.9 months]).
Conclusions: This study highlights the limitations of employing just one HER2 testing methodology in current clinical practice. It identifies a cohort of patients who did not receive potentially efficacious therapy because their tumor HER2-positivity was not determined by the test initially used. Because of inherent limitations in testing methodologies, it is inadvisable to rely on a single test to rule out potential benefit from HER2-targeted therapy.
Keywords: breast cancer; discordance rate; fluorescence in situ hybridization; human epidermal growth factor receptor 2; immunohistochemistry; local and central HER2 testing.
© 2014 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Figures

References
-
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neo oncogene. Science. 1987;235:177–182. - PubMed
-
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. - PubMed
-
- Estevez LG, Seidman AD. HER2-positive breast cancer: incidence, prognosis, and treatment options. Am J Cancer. 2003;2:169–179.
-
- Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

